Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
OP TANDON|Exercise OBJECTIVE TYPE QUESTION (LEVEL -A)|194 VideosELECTROCHEMISTRY
OP TANDON|Exercise OBJECTIVE TYPE QUESTION (LEVEL -B)|52 VideosELECTROCHEMISTRY
OP TANDON|Exercise ILLUSTRATIONS|28 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise SBJECTIVE TYPE|96 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Single Interger answer type questions|14 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ELECTROCHEMISTRY-PRACTICE PROBLEMS
- The chemical reaction given below : Cl(2)(g)+SO(2)(g) +2H(2)O(l) rar...
Text Solution
|
- A current of 80 microampere is passed through a solution of AgNO(3) fo...
Text Solution
|
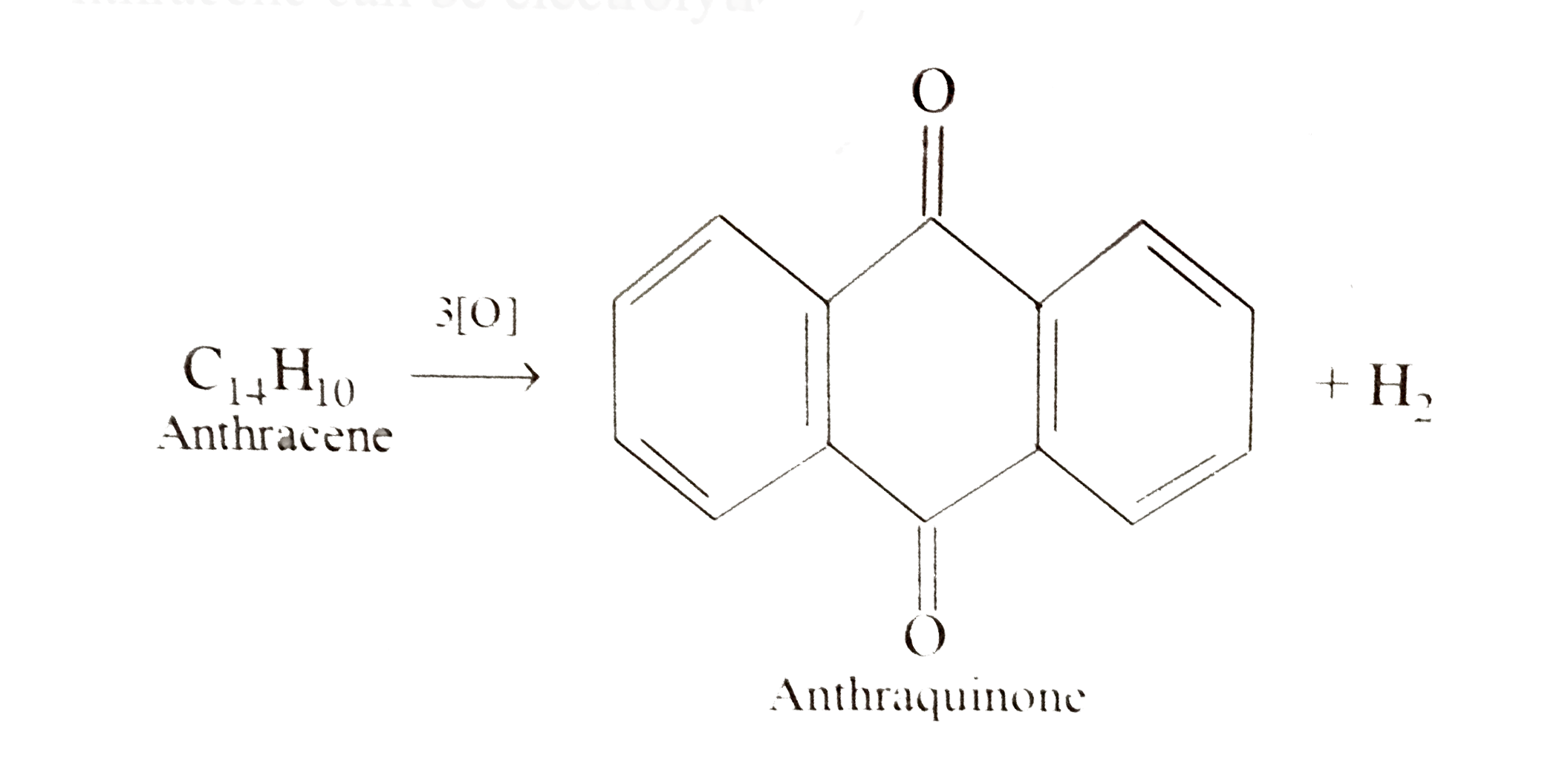
- Anthracene can be electrokytically oxidised to anthraquinone What...
Text Solution
|
- How many coulombs of electricity would be required to reduce the iron ...
Text Solution
|
- What current strength in ampere will be required to liberate 5 g of io...
Text Solution
|
- The mass of copper deposited from a solution of copper sulphate by a u...
Text Solution
|
- The solution of a salt of a metal of atomic mass 112 was electrolysed ...
Text Solution
|
- If a monovalent metal ion carries 1.6xx10^(-19) coulomb of electricity...
Text Solution
|
- Calculate aprooximately how much current is necessary to produce oxyge...
Text Solution
|
- 0.5 faraday of electricity was required to deposit all the copper in 5...
Text Solution
|
- Calculate the mass and volume at NTP of hydrogen and chlorine that wil...
Text Solution
|
- An electric current passing for 6 minutes through a dilute H(2)SO(4) s...
Text Solution
|
- The same quantity of electricity that liberated 2.158 g silver was pas...
Text Solution
|
- Calculate the volume of Cl(2) at NTP produced during electrolysis of M...
Text Solution
|
- How long does it take to deposit 100 g of Al from an electrolytic cell...
Text Solution
|
- 10g farily concentrated solution of CuSO(4) is electrolysed using 1.01...
Text Solution
|
- The density of copper is 8.94 g mL^(-1). Find out the number of coulom...
Text Solution
|
- What mass of Ag (At. Mass 108) could be plated on a spoon from electro...
Text Solution
|
- If a current of 0.3 ampere is drawn from a Daniell cell for 1 hour, wh...
Text Solution
|
- How many coulombs must be applied to a cell for the electrolytic produ...
Text Solution
|