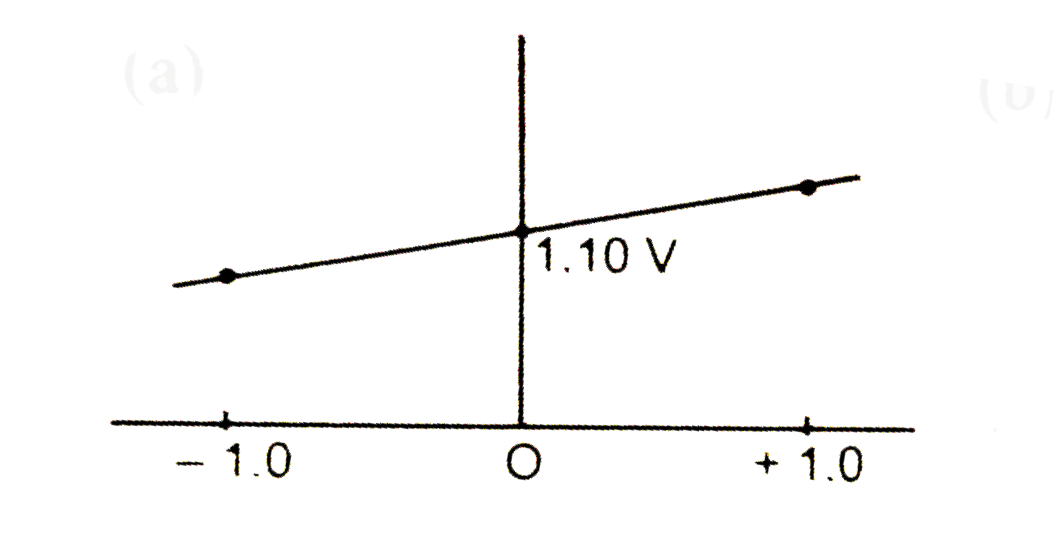
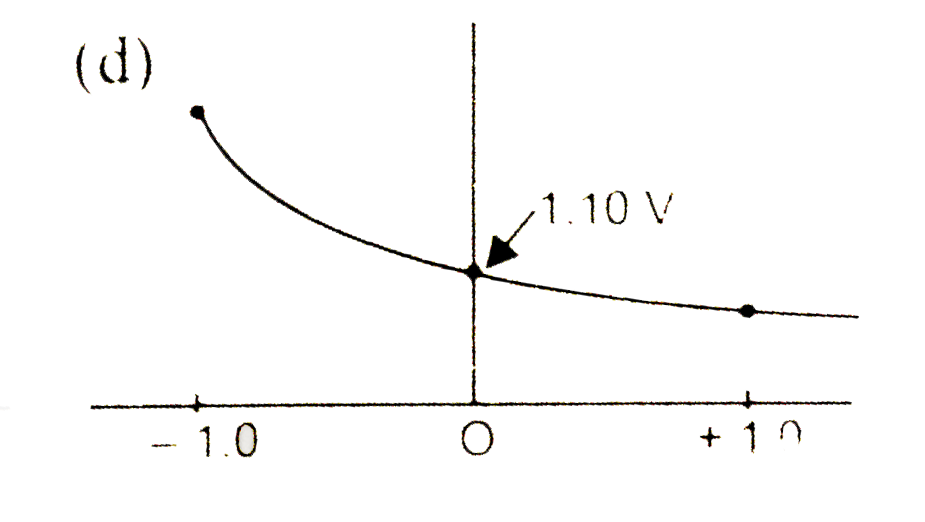
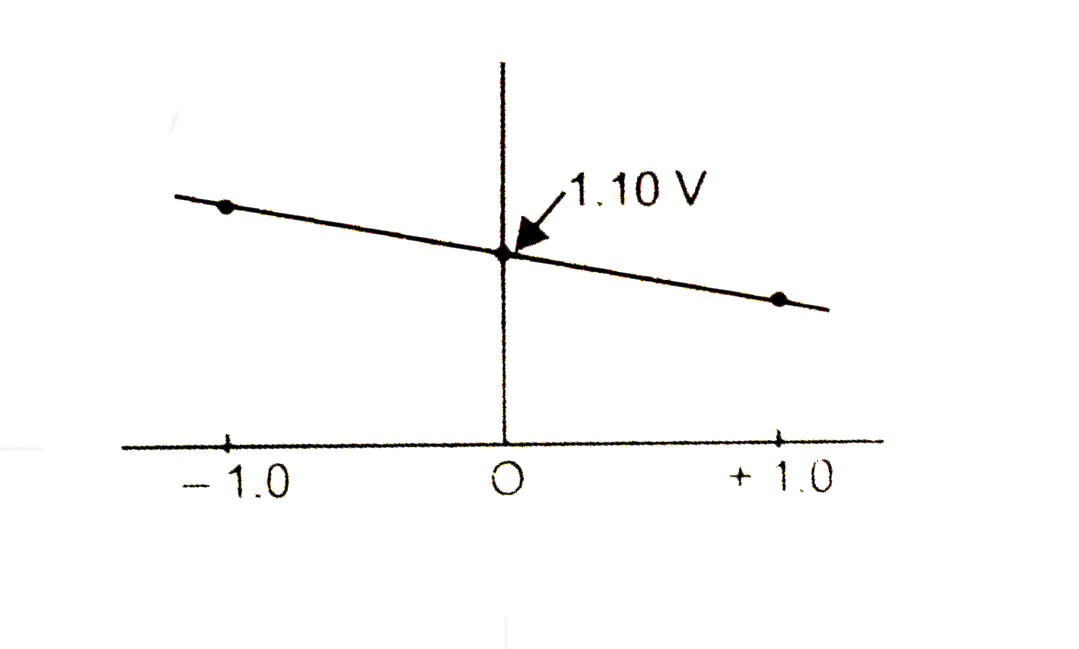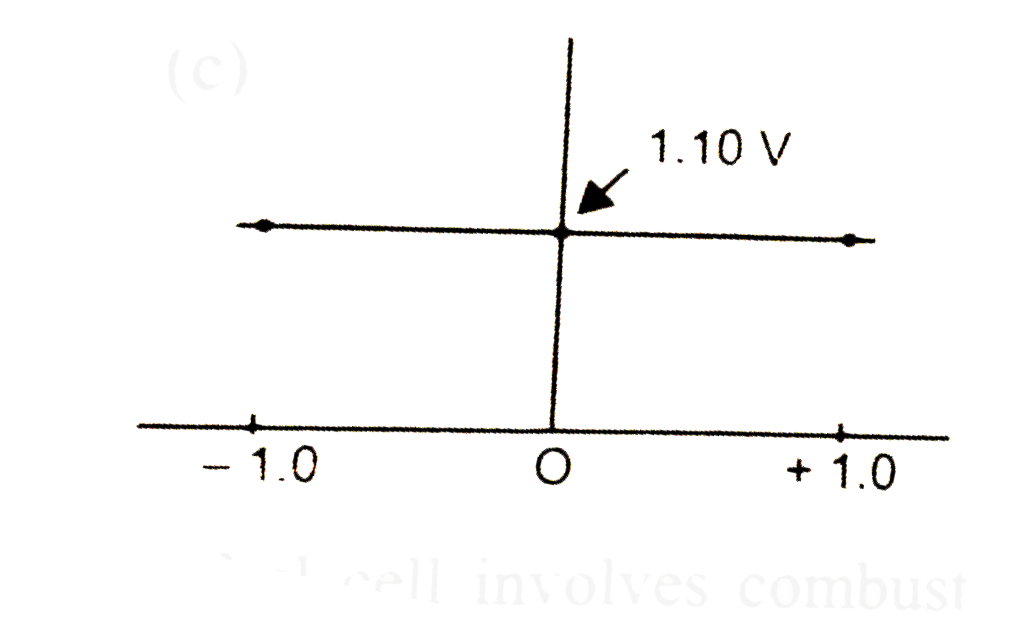A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
OP TANDON|Exercise One or more than correct answer|19 VideosELECTROCHEMISTRY
OP TANDON|Exercise Assertion- Reason|27 VideosELECTROCHEMISTRY
OP TANDON|Exercise OBJECTIVE TYPE QUESTION (LEVEL -A)|194 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise SBJECTIVE TYPE|96 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Single Interger answer type questions|14 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ELECTROCHEMISTRY-OBJECTIVE TYPE QUESTION (LEVEL -B)
- The efficiency of a hypothetical cell is about 84% which involves the ...
Text Solution
|
- A chemical reaction will be non-spontaneous if
Text Solution
|
- Match the List-I with List-II {:(,"List-I (Electrode)",,"List -II (T...
Text Solution
|
- The conductivity of saturated solution of BaSO(4) is 3.06xx10^(-6) ohm...
Text Solution
|
- A standard hydrogen electrode has zero electrode potential because :
Text Solution
|
- I. Conductance of electolyte solution increases with temperature II....
Text Solution
|
- I. The conductivity of molten NaCl is due to movement of Na^(+) and Cl...
Text Solution
|
- I. Cathode is -ve terminal both in electrochemical and electrolytic ce...
Text Solution
|
- In an experiment, 0.04 F was passed through 400 mL of a 1M solution of...
Text Solution
|
- An alloy of Pb-Ag weighing 1.08g was dissolved in dilute HNO(3) and th...
Text Solution
|
- Select the incorrect statements about dry cell:
Text Solution
|
- Given the standard oxidation potentials Fe overset(+0.4V)rarr Fe^(2+...
Text Solution
|
- Dipping iron article into a strongly alkaline solution of sodium phosp...
Text Solution
|
- For the redox process Zn (s) + Cu^(2+) hArr Zn^(2+) + Cu(s) E("cell"...
Text Solution
|
- C(4)H(10)+(13)/(2)O(2)(g)rarr4CO(2)(g)+5H(2)O(l), DeltaH =- 2878 kJ ...
Text Solution
|
- Molar conductance Lamda(m) is plotted against sqrt(C) (mol "litre"^(-1...
Text Solution
|
- In the concentration cell Pt (H(2)) |(HA (0.1M)),(Na A (1M))||(HA (1...
Text Solution
|
- In the following process of disproportionation: underset(underset("i...
Text Solution
|
- For the following electrochemical cell at 298 K, pt (s) |H(2) (g, 1 ...
Text Solution
|
- For the following cell, Zn(s)|ZnSO(4)(aq)||CuSO(4)(aq)||Cu(s) When...
Text Solution
|