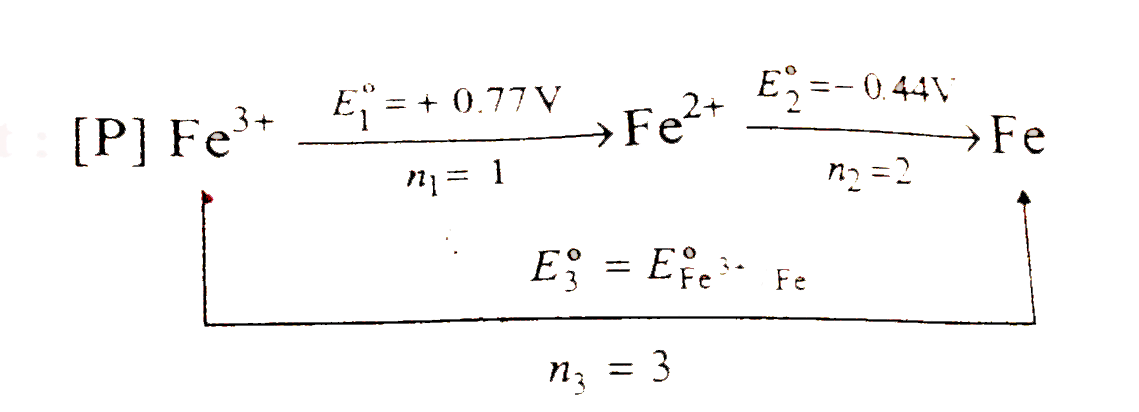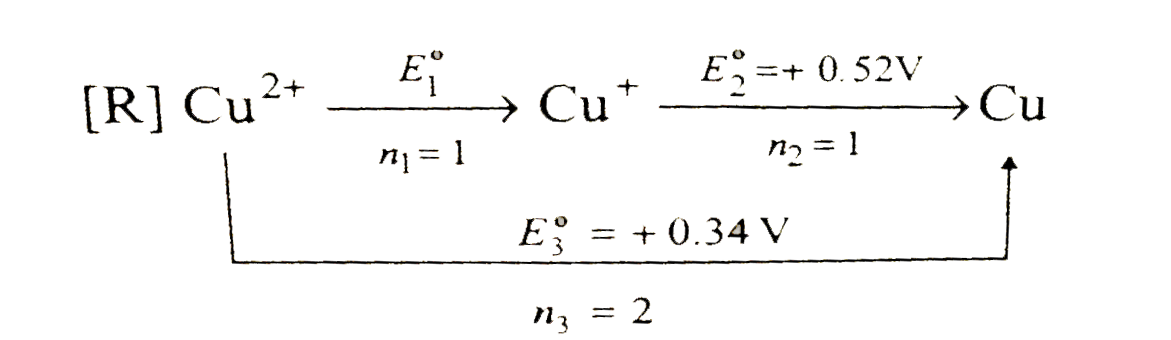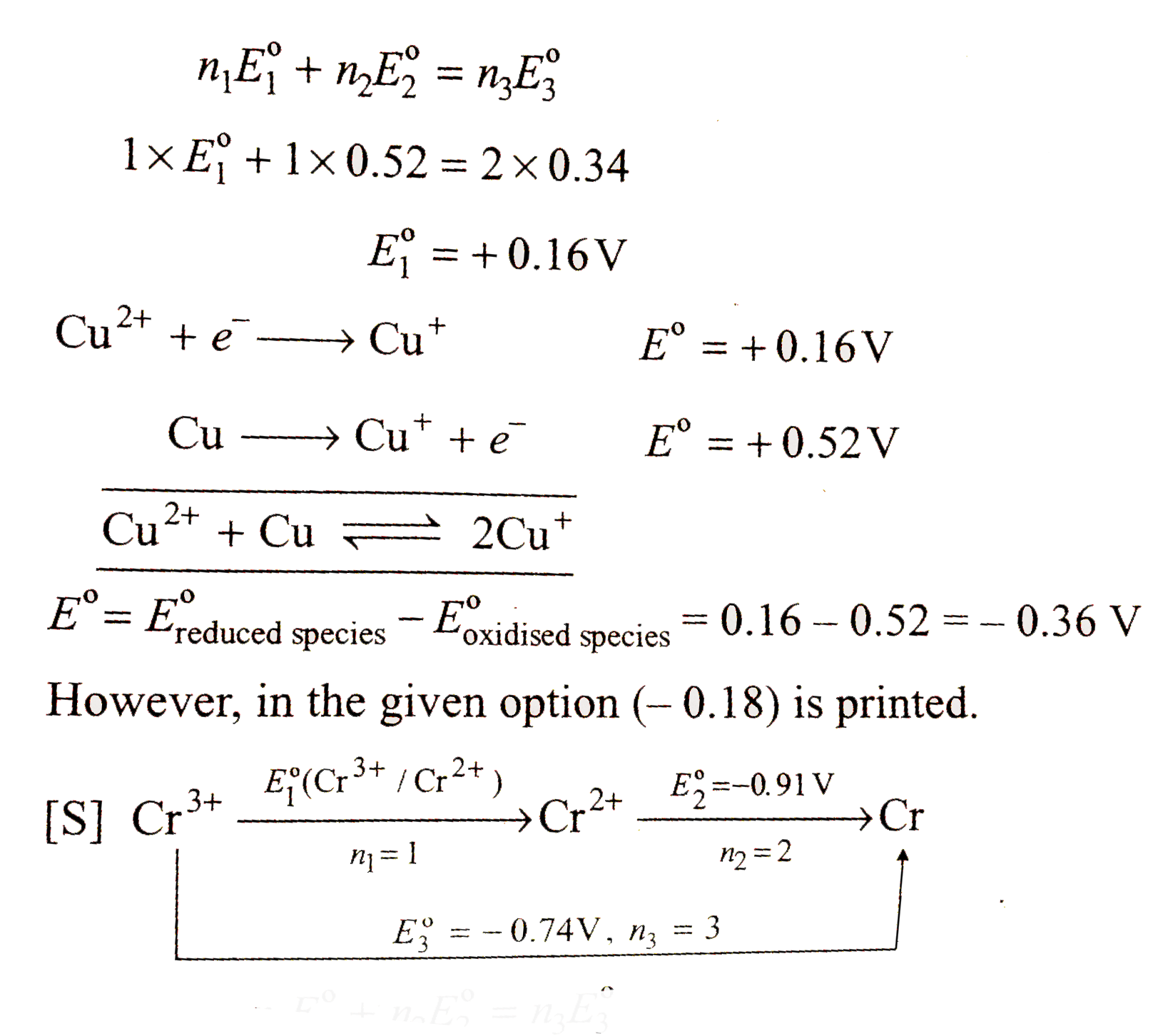A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
OP TANDON|Exercise Integer Type Question|16 VideosELECTROCHEMISTRY
OP TANDON|Exercise LINKED COMPREHENSION TYPE QUESTION|40 VideosELECTROCHEMISTRY
OP TANDON|Exercise Assertion- Reason|27 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise SBJECTIVE TYPE|96 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Single Interger answer type questions|14 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ELECTROCHEMISTRY-Matching Type Question
- Match the List -I with List -II and List -III :
Text Solution
|
- Match the salts in the Column-I with their use in Column -II :
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|
- Match the physical quantities in the List-I with their units in List-I...
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|
- The standard reduction potential data at 25^(@)C is given below E^(@...
Text Solution
|