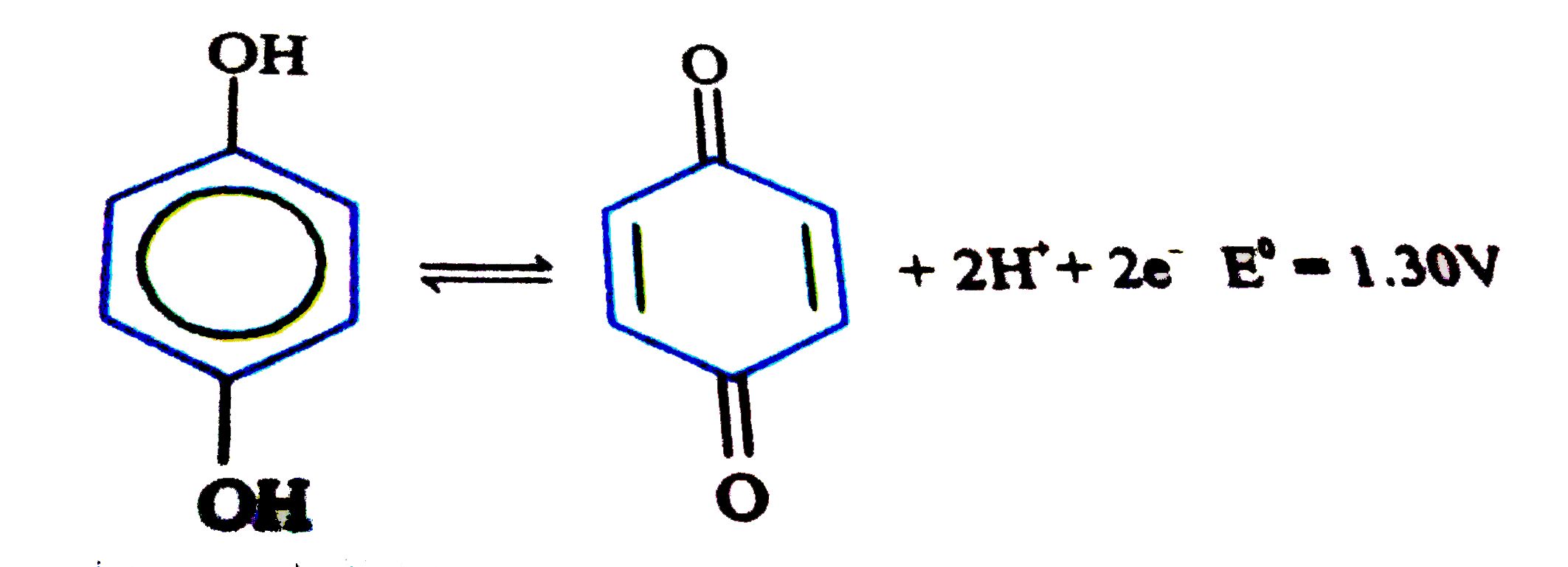A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
OP TANDON|Exercise Multiple Answer Type Question|5 VideosELECTROCHEMISTRY
OP TANDON|Exercise Matrix-Matching Type Question|3 VideosELECTROCHEMISTRY
OP TANDON|Exercise LINKED COMPREHENSION TYPE QUESTION|40 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise SBJECTIVE TYPE|96 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Single Interger answer type questions|14 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ELECTROCHEMISTRY-Straight Objective Type Questions
- The specific conductance (kappa) of an electrolyte of 0.1 N concentrat...
Text Solution
|
- The standard E("Red")^(@) values of A,B,C are 0.68V, - 2.54V,- 0.50V...
Text Solution
|
- In the electrochemical reaction, 2Fe^(3+)+Zn rarr Zn^(2+) + 2Fe^(2+)...
Text Solution
|
- Fully charges lead storage battery contains 1.5 L of 5 M H(2)SO(4). If...
Text Solution
|
- In a gydrogen - oxygen fuel cell, 67.2 litre of H(2) at S.T.P is used ...
Text Solution
|
- For the half cell At pH = 3 electrode potential is
Text Solution
|
- The standard reduction potentials E^(@) for OCl^(-)//Cl^(-) and for Cl...
Text Solution
|
- The standard reduction potential for two reactions are given below A...
Text Solution
|
- The equilibrium constant for the reaction, Cu(s)+Cu^(2+) (aq.) hArr ...
Text Solution
|
- Cations absorb 6.023xx10^(22) electrons for their reduction. How many ...
Text Solution
|