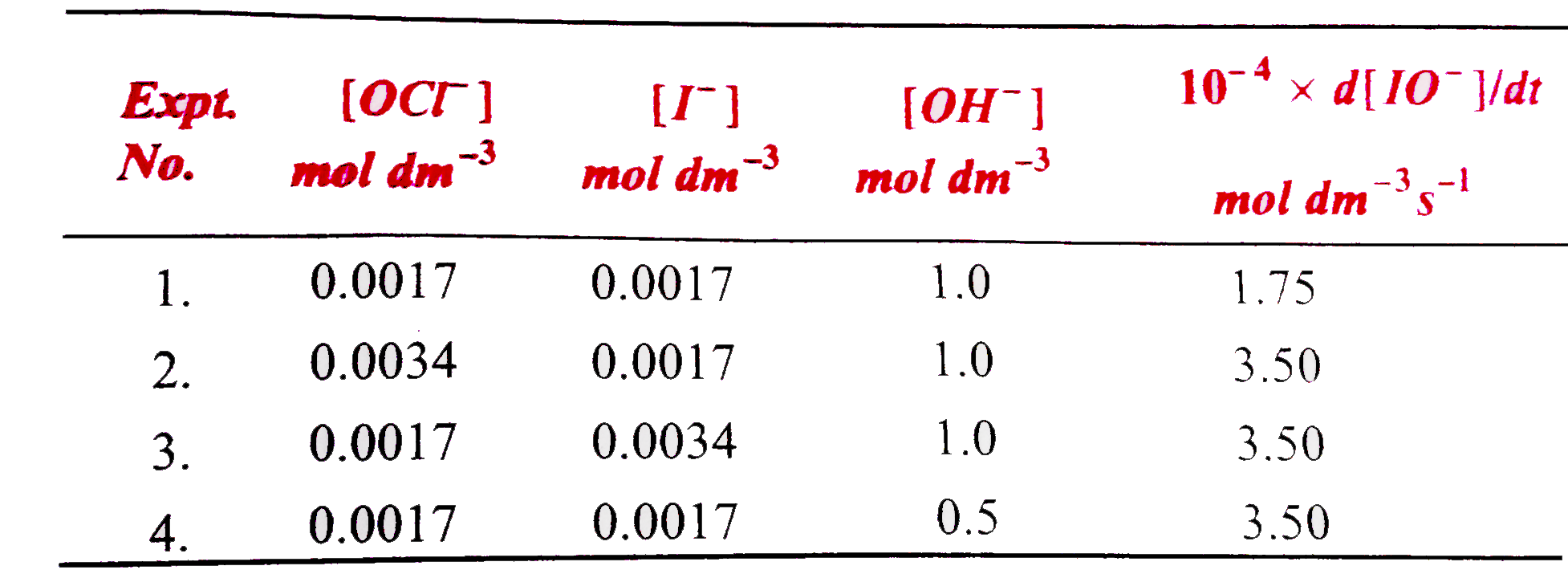Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)
- The table given below gives kinetic data for the following reaction at...
Text Solution
|
- The rate of chemical reaction are strongly affected by temperature . A...
Text Solution
|
- The rate of chemical reaction are strongly affected by temperature . A...
Text Solution
|
- The rate of chemical reaction are strongly affected by temperature . A...
Text Solution
|
- The rate of chemical reaction are strongly affected by temperature . A...
Text Solution
|