A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise PRACTICE PROBLEMS|70 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-A)|192 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-ILLUSTRATIONS
- For the decompoistion of HI at 1000 K(2HI rarr H(2)+I(2)), following d...
Text Solution
|
- Conisder a reaction A rarr B + C . The initial concentration of A was ...
Text Solution
|
- The rate of a gaseous reaction is given by the expresison k[A]^(2)[B]^...
Text Solution
|
- For a chemical reaction A rarr B, the rate of reaction increases by a ...
Text Solution
|
- The rate of the reaction 3A + 2B rarr Products is given by the rat...
Text Solution
|
- For the reaction A + Brarr C +D, if concentratiton of A is doubled wit...
Text Solution
|
- Inversion of can sugar in dilute acid (conversion into glucose and fru...
Text Solution
|
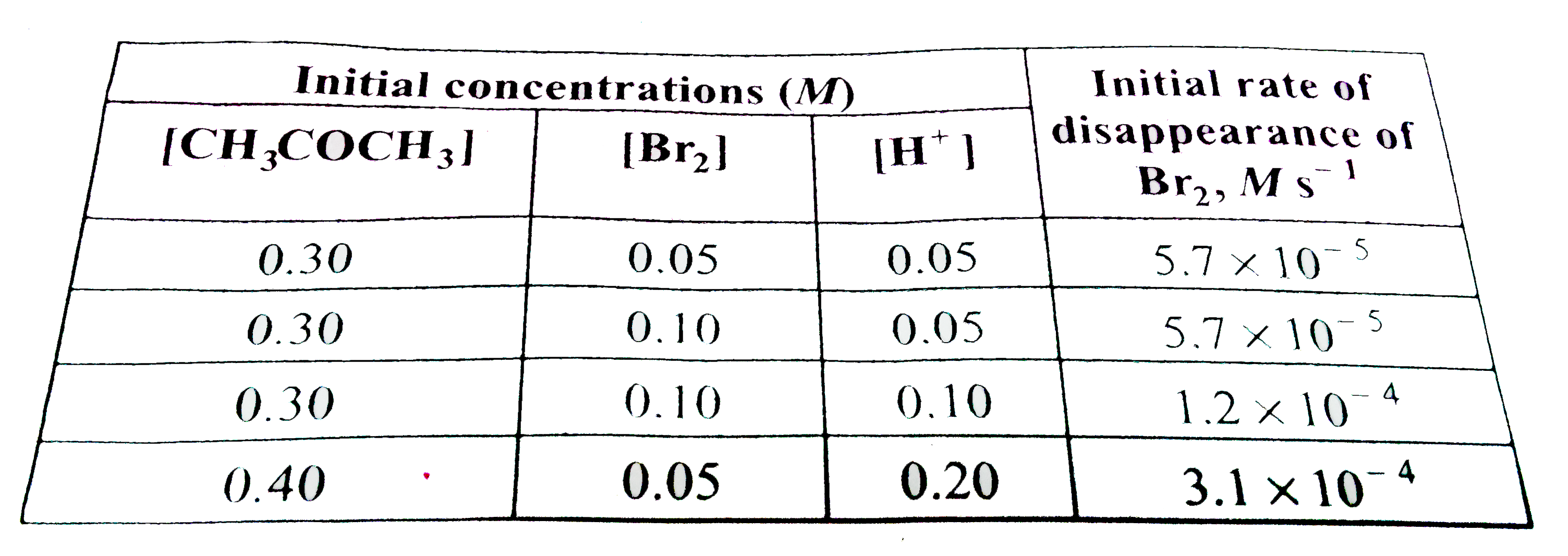
- The bromination of acetone that occurs in acid solution is represente...
Text Solution
|
- A first order reaction has half life of 14.5 hrs. What percentage of t...
Text Solution
|
- Half life of a first order reaction in 10 min. What % of reaction will...
Text Solution
|
- A certain zero order reaction has k = 0.025 M s^(-1) for the disappear...
Text Solution
|
- The first order reaction 2N(2)O(g)rarr2N(2)(g)+O(2)(g) has a rate cons...
Text Solution
|
- The reaction of O(3) with chlorine atom is given as : O(3)(g)+Cl(g)r...
Text Solution
|
- If a first order reaction takes 32 minutes for 75% completion, then ti...
Text Solution
|
- Rate constant of a reaction (k) is 175 "litre"^(2) "mol"^(-2)sec^(-1)....
Text Solution
|
- The half life of a first order reaction having rate constant k=1.7xx10...
Text Solution
|
- At 500 K, the half-life period of a gaseous reaction at the initial pr...
Text Solution
|
- 90% of the first order reaction is completed in 70 minutes. The veloci...
Text Solution
|
- The half life period of a first reaction is 1 min 40 seconds. Calulate...
Text Solution
|
- At T (K) , if the rate constant of a first order reaction is 4.606xx1...
Text Solution
|