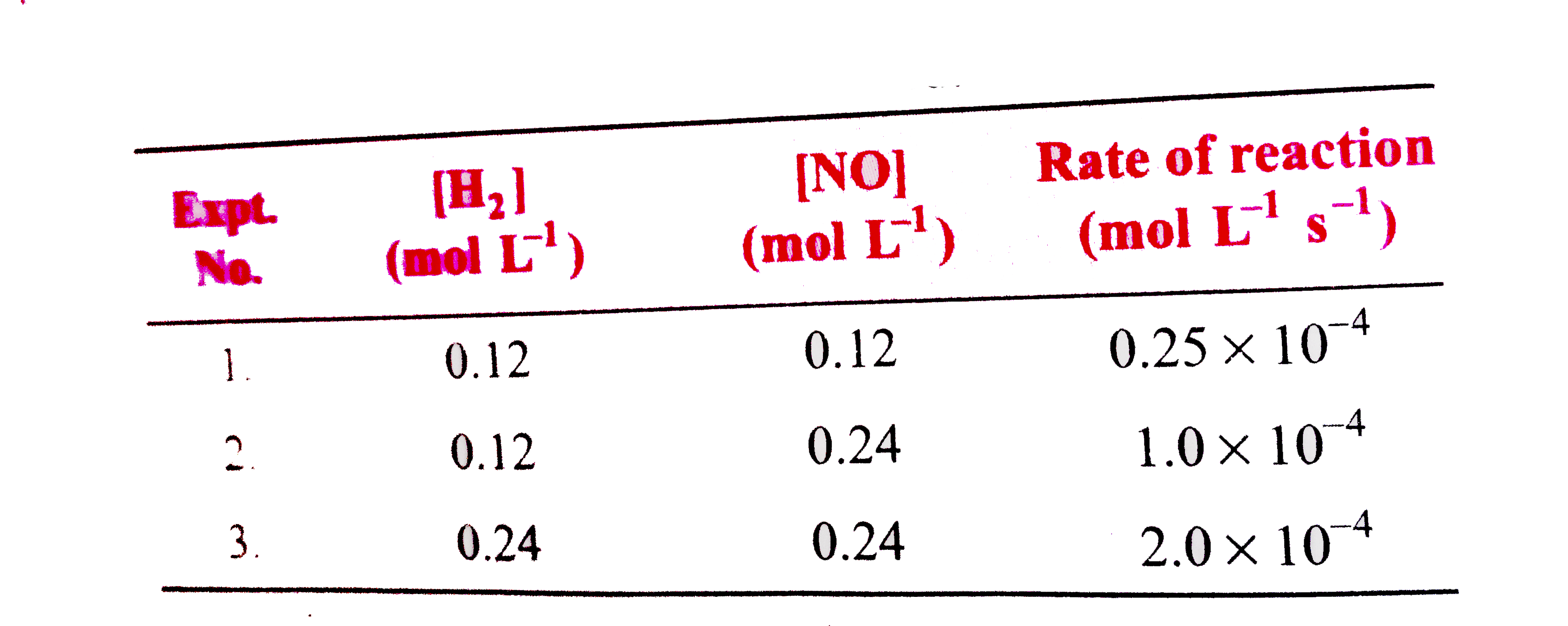Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-A)|192 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET I|48 VideosCHEMICAL KINETICS
OP TANDON|Exercise ILLUSTRATIONS|34 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-PRACTICE PROBLEMS
- What is the rat law expressio for the reaction , A+BrarrC The foll...
Text Solution
|
- The reaction , 2N(2)O(5)rarr4NO(2)+O(2) was studdied and the foll...
Text Solution
|
- For the reaction, 2NO+2H(2)rarrN(2)+2H(2)O, the following kinetic ...
Text Solution
|
- Fill in the balanks in the following table which treats reaction of a ...
Text Solution
|
- The reaction 2NO+O(2)rarr2NO(2) follows the rate law =k[NO]^(2)[O(2)]...
Text Solution
|
- From the following data of initial concentration and rates, calculate ...
Text Solution
|
- The following initial rate data were obtianed for the reaction 2NO...
Text Solution
|
- The data given below are for the reaction of NO and Cl(2) to from NOCl...
Text Solution
|
- In the hydrogen of propyl acetate in presence of dilute HCl in aqueous...
Text Solution
|
- In a first order reaction the concentration of the reactant is reduced...
Text Solution
|
- For a first order reaction, the rate constant is 0.1 s^(-1). How much ...
Text Solution
|
- A substance decomposes following first order reaction. If the half lif...
Text Solution
|
- In a first order reaction the concentration of the reactant is reduced...
Text Solution
|
- Find the two-thirds life (t(2//3)) of a first order reaction in which ...
Text Solution
|
- A first order reaction has a specific rate of 10^(-3) "sec"^(-1) . How...
Text Solution
|
- In a hydrolysis reaction, 5 g of ethyl acetate is hydroloysed in the p...
Text Solution
|
- Calculate the half life of the reaction ArarrB, when the initial conce...
Text Solution
|
- In reaction, ArarrB +C the following data were obtained. {:("t in se...
Text Solution
|
- The first order reaction has k=1.5xx10^(-6) per second at 200^(@)C . I...
Text Solution
|
- What will be the initial rate of a reaction if its rate constant is 1...
Text Solution
|