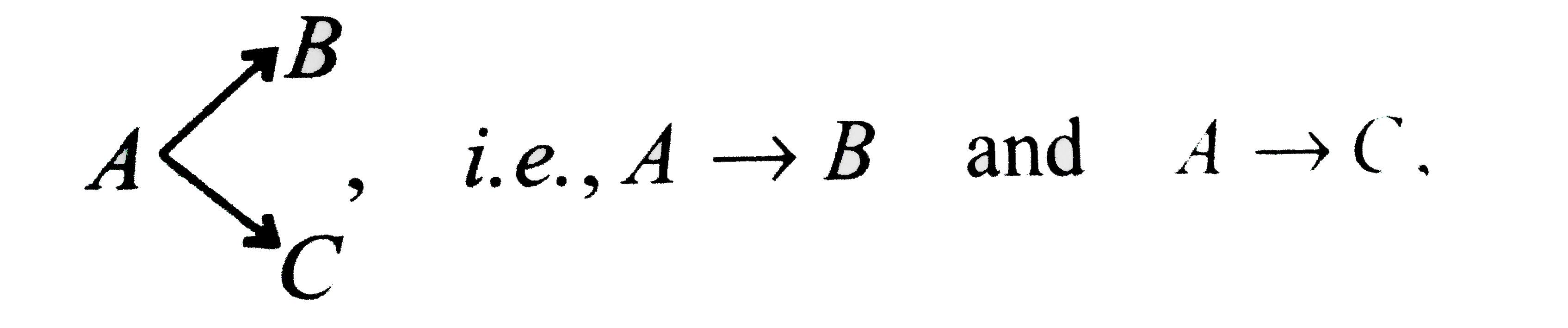
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-A)|192 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET I|48 VideosCHEMICAL KINETICS
OP TANDON|Exercise ILLUSTRATIONS|34 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-PRACTICE PROBLEMS
- Given the following graph. (a) Calculate Delta H for the reaction an...
Text Solution
|
- Temperature coceficient, mu=(k(35^(@)C))/(k(25^(@)C)) of a reaction is...
Text Solution
|
- For first order gaseous reaction , log k when plotted atgains (1)/(t) ...
Text Solution
|
- The rate at 27^(@)C of a chemical reaction increases 1000 times when a...
Text Solution
|
- Dehydration of tertiary butyl alcohol follows a first order reaction, ...
Text Solution
|
- Let us consider the following mechanism : CH(3)CN+^(+)hArrCH(3)CNH^(...
Text Solution
|
- The thermal isomerization of cyclopropane occures according to the equ...
Text Solution
|
- A substance '' was found to undergo two parallel first order reaction ...
Text Solution
|
- Two reactions proceed at 25^(@)C at the same rate, the temperature coe...
Text Solution
|
- Half lives against initial pressure are given below. Calculate the ord...
Text Solution
|
- for a given reaction at temeperature T, the velocity constant k, is ex...
Text Solution
|
- If the activation energy of a reaction is 80.9 kJ "mol"^(-1). Calculat...
Text Solution
|
- The following data wer obtained for a given reaction at 300 K Cal...
Text Solution
|
- The Arrhenius equations for cis-trans isomerzation of but-2-ene (CH(3)...
Text Solution
|
- The half life for a reaction between fixed conecntration of reactants ...
Text Solution
|
- What percentage of reactant molecules will crossover the energe barrie...
Text Solution
|
- In milk at 37^(@)C , lactobalicillus acidophilus has a generation time...
Text Solution
|
- Two reactiosn of same order have equal pre-exponential factors but the...
Text Solution
|
- A decompositio has following mecfhanism 2N(2)O(5)rarr4NO(2)+" ...
Text Solution
|
- Rate constant of a reaction changes by 2% by 0.1^(@)C rise in temperat...
Text Solution
|