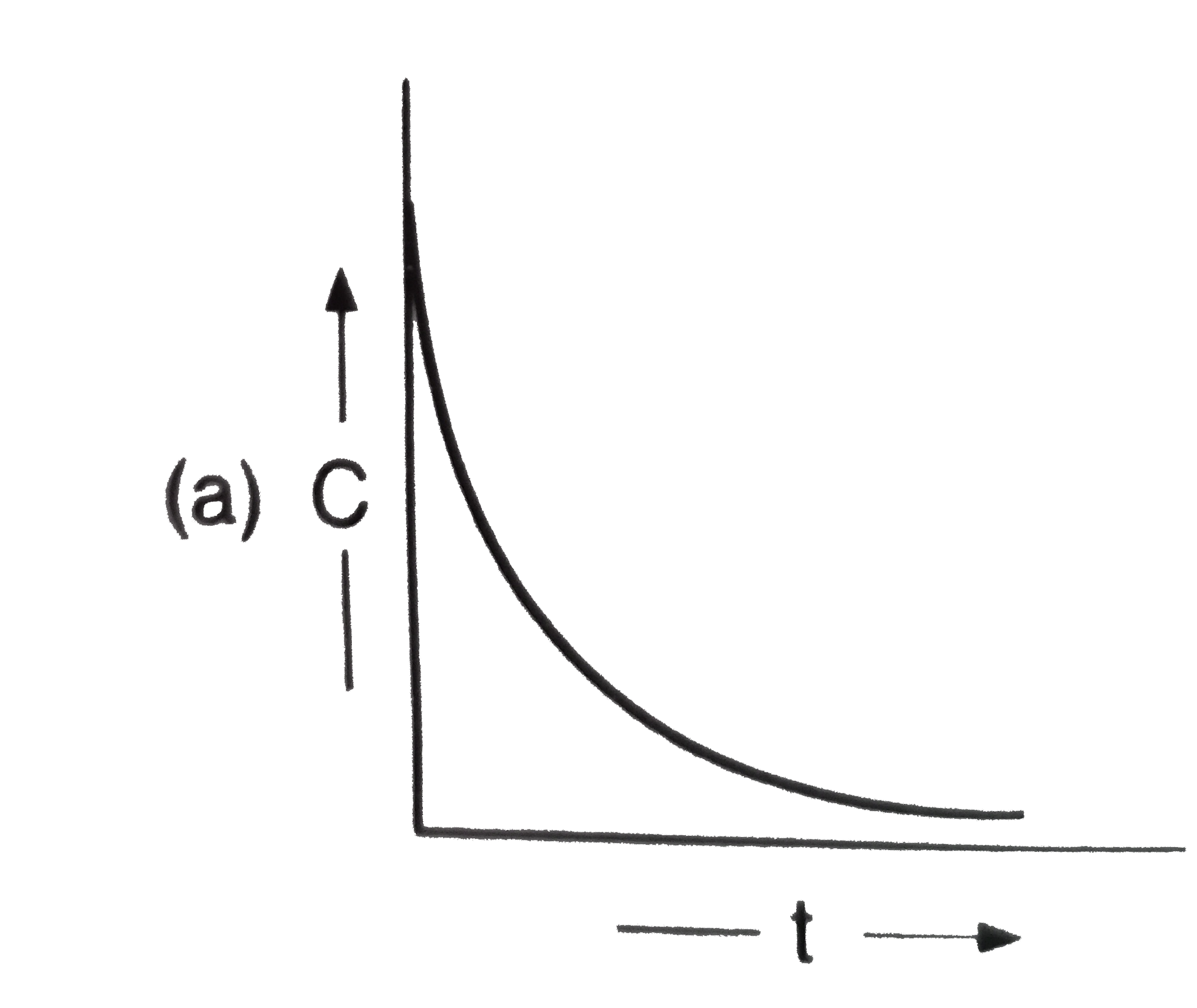
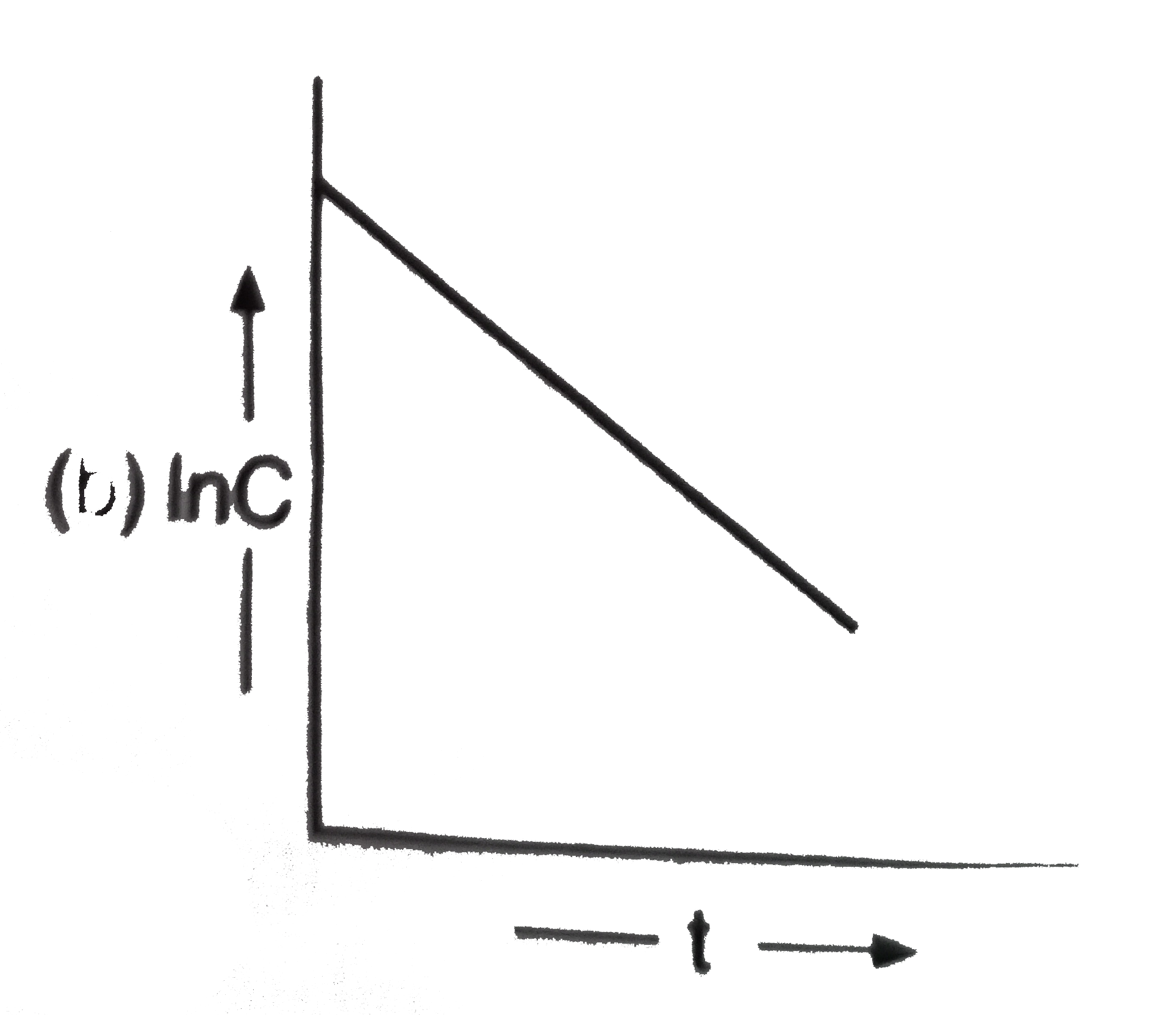
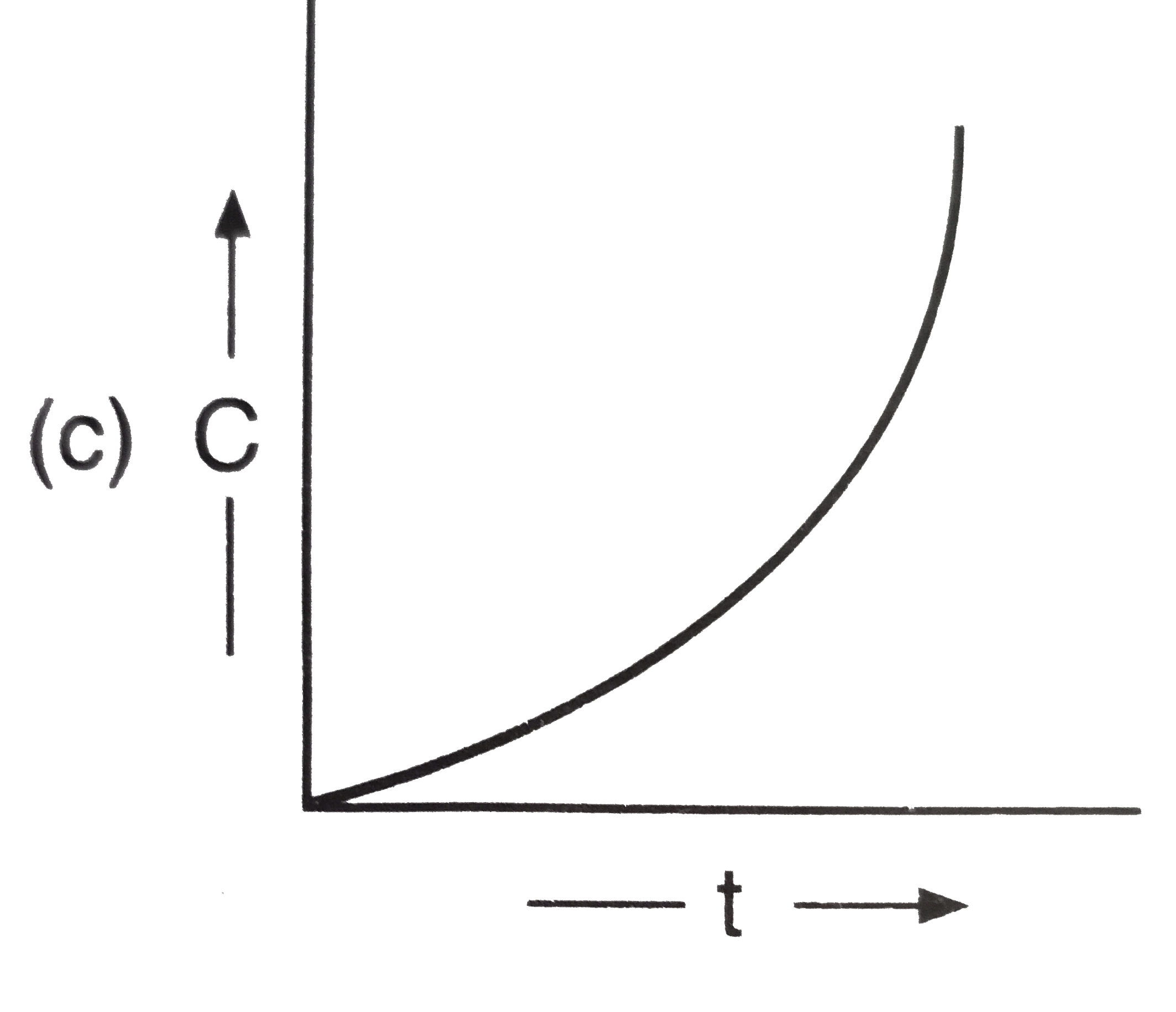
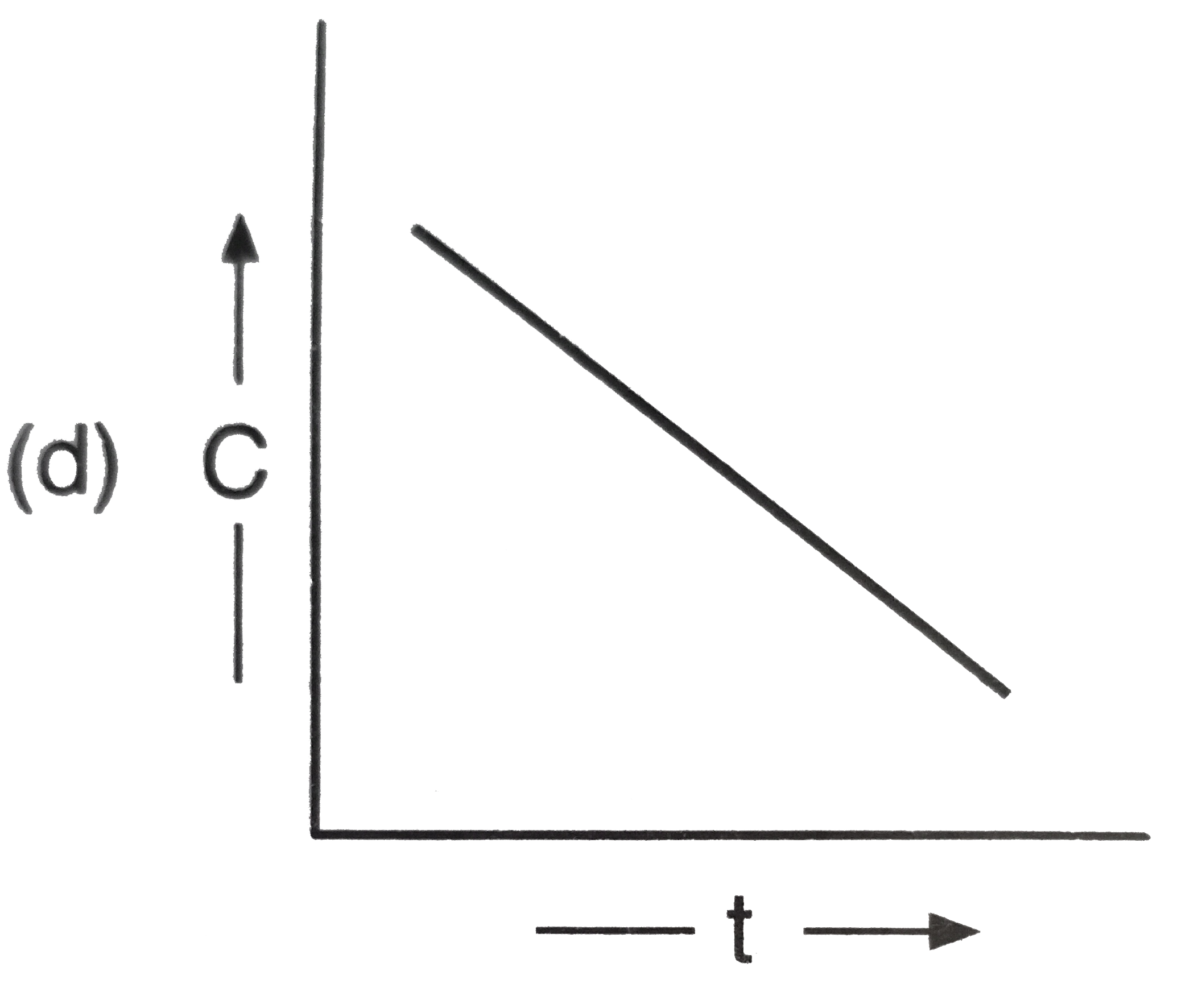
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET I|48 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET II|13 VideosCHEMICAL KINETICS
OP TANDON|Exercise PRACTICE PROBLEMS|70 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-OBJECTIVE QUESTIONS (LEVEL-A)
- The activation energy of a reaction can be lowered by :
Text Solution
|
- The activation energy for a hypothetical reaction A rarr X is 12.49 kc...
Text Solution
|
- The plote between concentration versus time for a zero order reaction ...
Text Solution
|
- The rate of reaction increases with rise in temerature because of :
Text Solution
|
- Which of the following is a first order reaction ?
Text Solution
|
- Which one of the following is not a first order reaction?
Text Solution
|
- Rate expression of a chemical change is -(dx)/(dt)=k[A]^(2)[B]^(1)[C]^...
Text Solution
|
- For a reaction 2A +B rarr C+D, the active mass of B is kept constant b...
Text Solution
|
- The rate of certain hypothetical reaction A+B+C rarr Products, is gi...
Text Solution
|
- Which of the following rate laws has an overall order of 0.5 for react...
Text Solution
|
- If the rate of reaction between A and B is given by rate =k[A][B]^(2) ...
Text Solution
|
- For a reaction A rarr B, the rate of reaction quadrupled when the conc...
Text Solution
|
- For a chemical reactin ArarrE, it is found that rate of reaction is do...
Text Solution
|
- The rate of reaction A + B rarr Product is given by the equation r = k...
Text Solution
|
- The rate of reaction between A and B increases by a factor of 100, whe...
Text Solution
|
- The rate law for the reaction RCl + NaOH(aq) rarr ROH + NaCl is give...
Text Solution
|
- A zero order reactio is one:
Text Solution
|
- The rates of a certain at differnet time intervals are as follows : ...
Text Solution
|
- For which of the following reactions, the units of rate constant and r...
Text Solution
|
- The unite of rate constant for zero order reaction is :
Text Solution
|