A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET I|48 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET II|13 VideosCHEMICAL KINETICS
OP TANDON|Exercise PRACTICE PROBLEMS|70 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-OBJECTIVE QUESTIONS (LEVEL-A)
- In the reaction BrO^(-3)(aq) + 5Br^(-) (aq) + 6H^(+) rarr 3Br(2)(1) ...
Text Solution
|
- K is rate constant at temp T then value of underset(Ttooo)lim log K i...
Text Solution
|
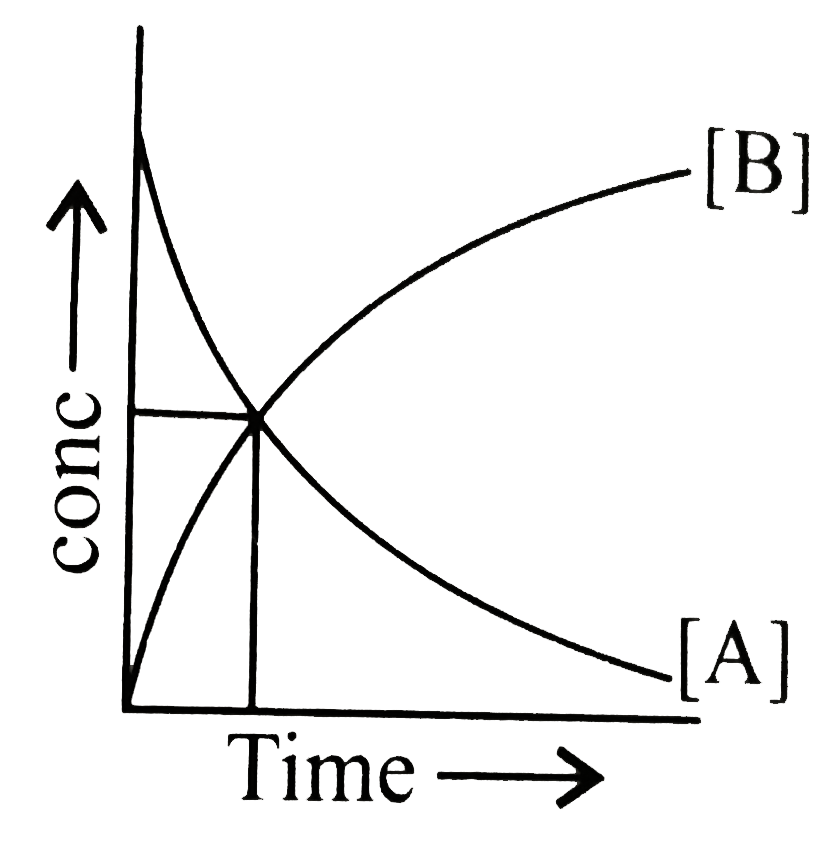
- The accompanying figure depicts a change in concentration of species A...
Text Solution
|
- In gaseous reaction, important for the understanding of the upper atmo...
Text Solution
|
- From the following data, the activation, energy for the reaction, (cal...
Text Solution
|
- The hydrolysis of an ester was carried out separately with 0.05 N HCl ...
Text Solution
|
- For an endothermic reaction where DeltaH represent the enthalpy of rea...
Text Solution
|
- In the following first order reactions: A+ "Reagent" overset(K(1))(r...
Text Solution
|
- Two reactions A rarr products and B rarr products have rate constants ...
Text Solution
|
- The inversion of a sugar follows first order rate equation which can b...
Text Solution
|
- The number of molecules of the reactants taking part in a single step ...
Text Solution
|
- The inversion of cane sugar into glucose and frucose is :
Text Solution
|
- The unit of rate constant obeying the rate expression r=k[A]'[B]^(2//3...
Text Solution
|
- For the reaction, N(2)O(5) rarr 2NO(2)+O(2), Given -(d[N(2)O(5)])/...
Text Solution
|
- The rate constant is unmerically the same for three reactions of first...
Text Solution
|
- In the Q.No , 108 if the concentration of the reactant is less than 1...
Text Solution
|
- In the Q.No 108 , if the concentration of the reactant is 1 M , then ...
Text Solution
|
- A first order reaction is one-fifth completed in 40 minutes. The time ...
Text Solution
|
- The rate constant off a first order reaction, Ararr"Products", is 60...
Text Solution
|
- For first order reaction, the half life is dependent of :
Text Solution
|