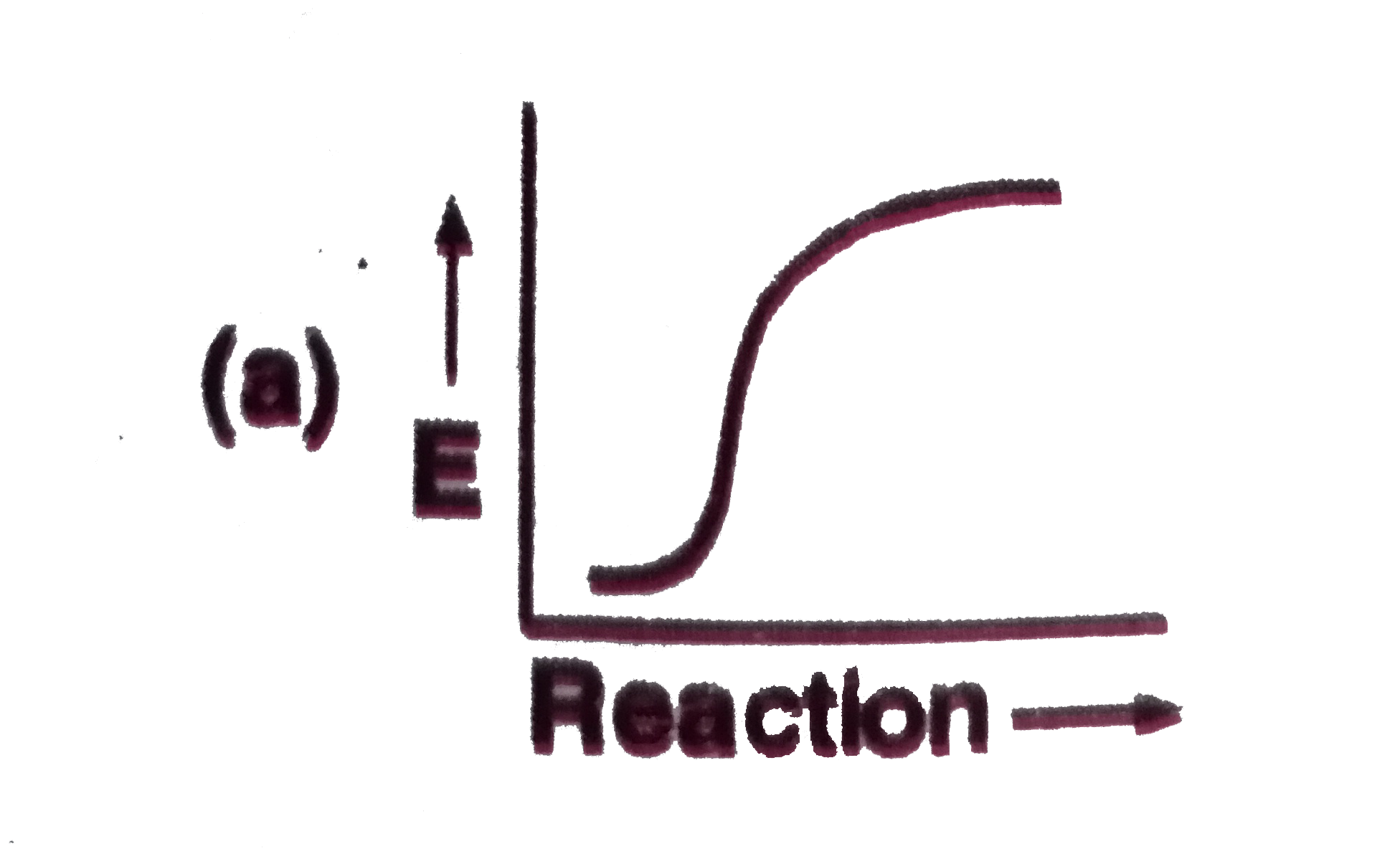
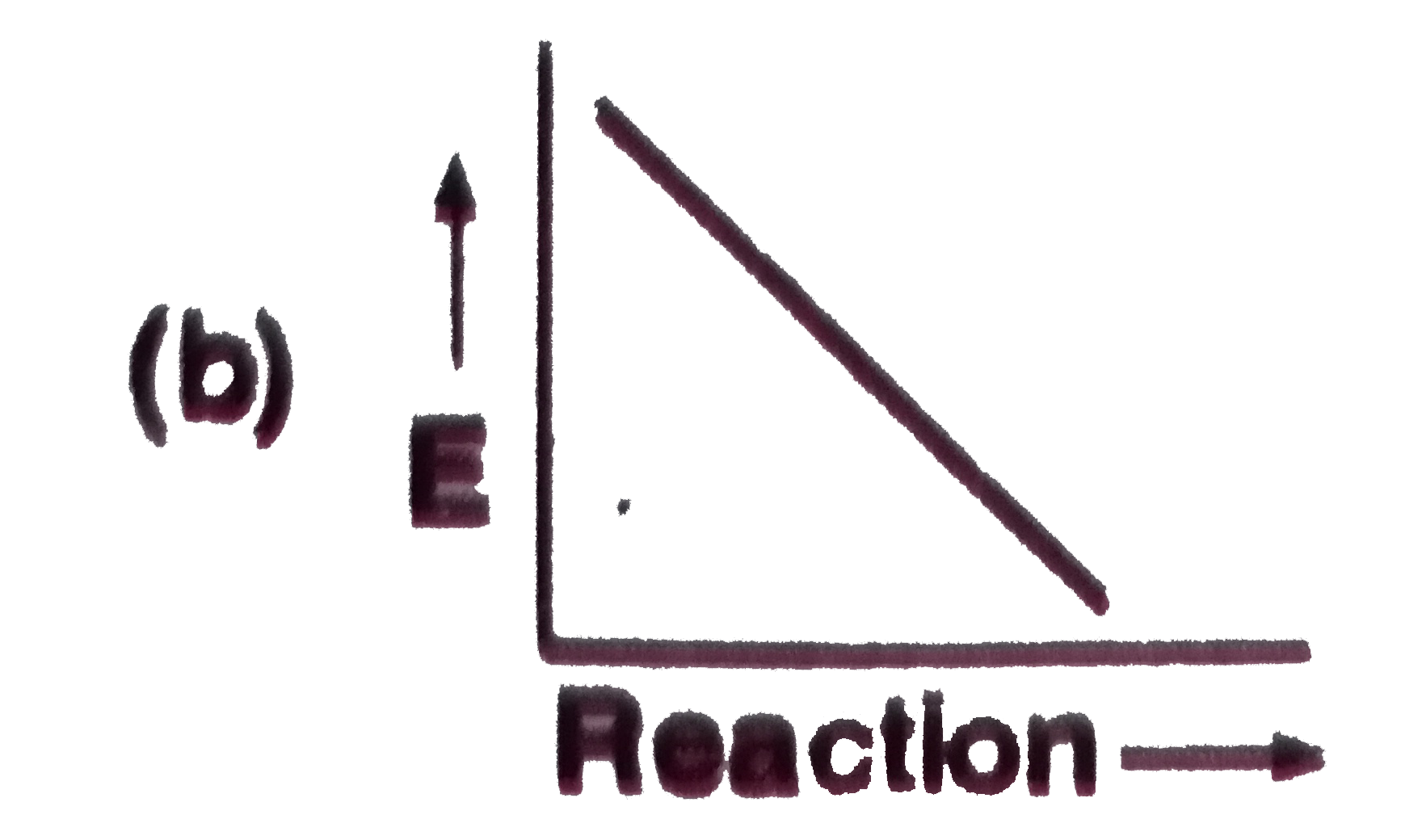
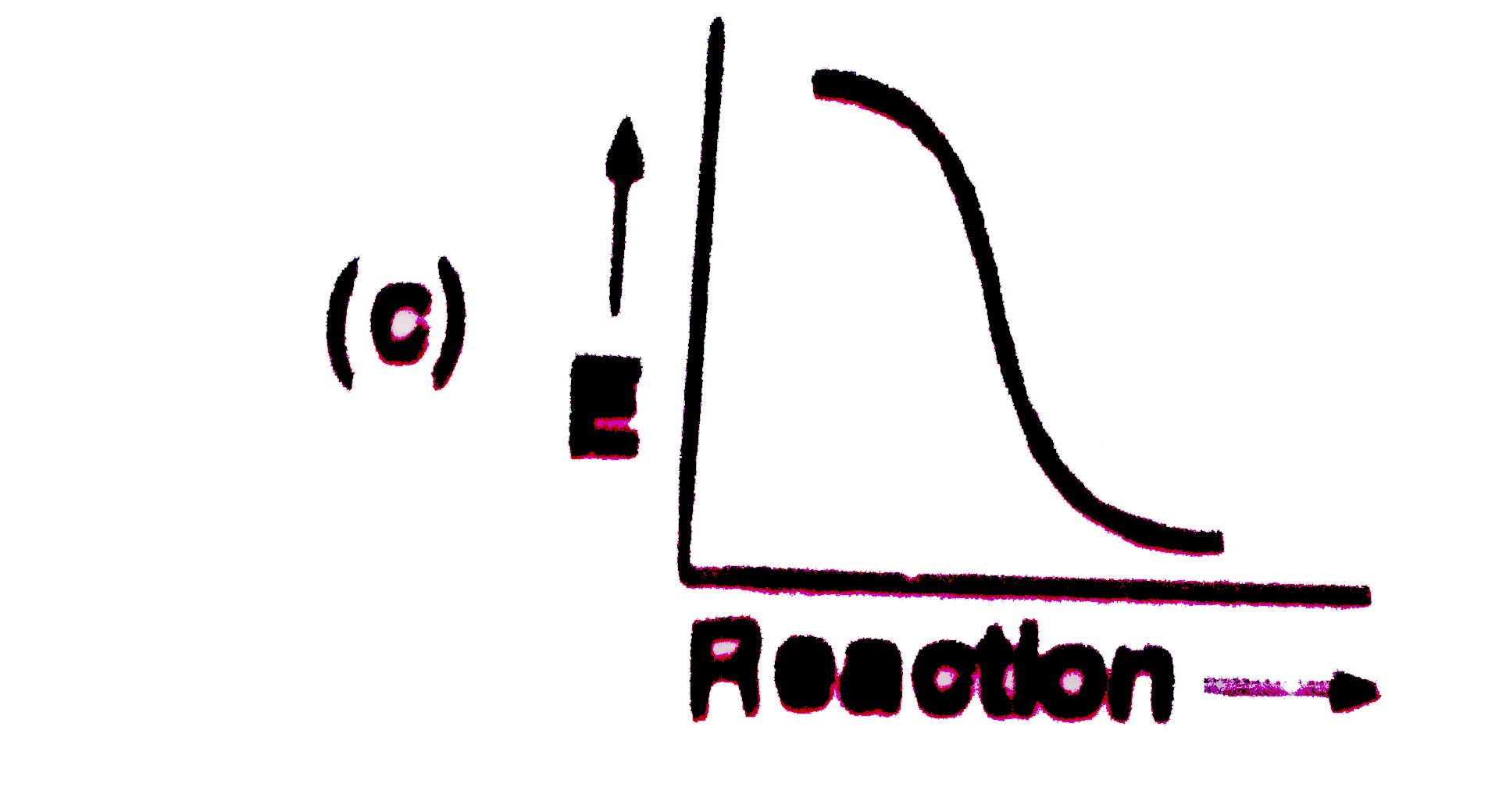
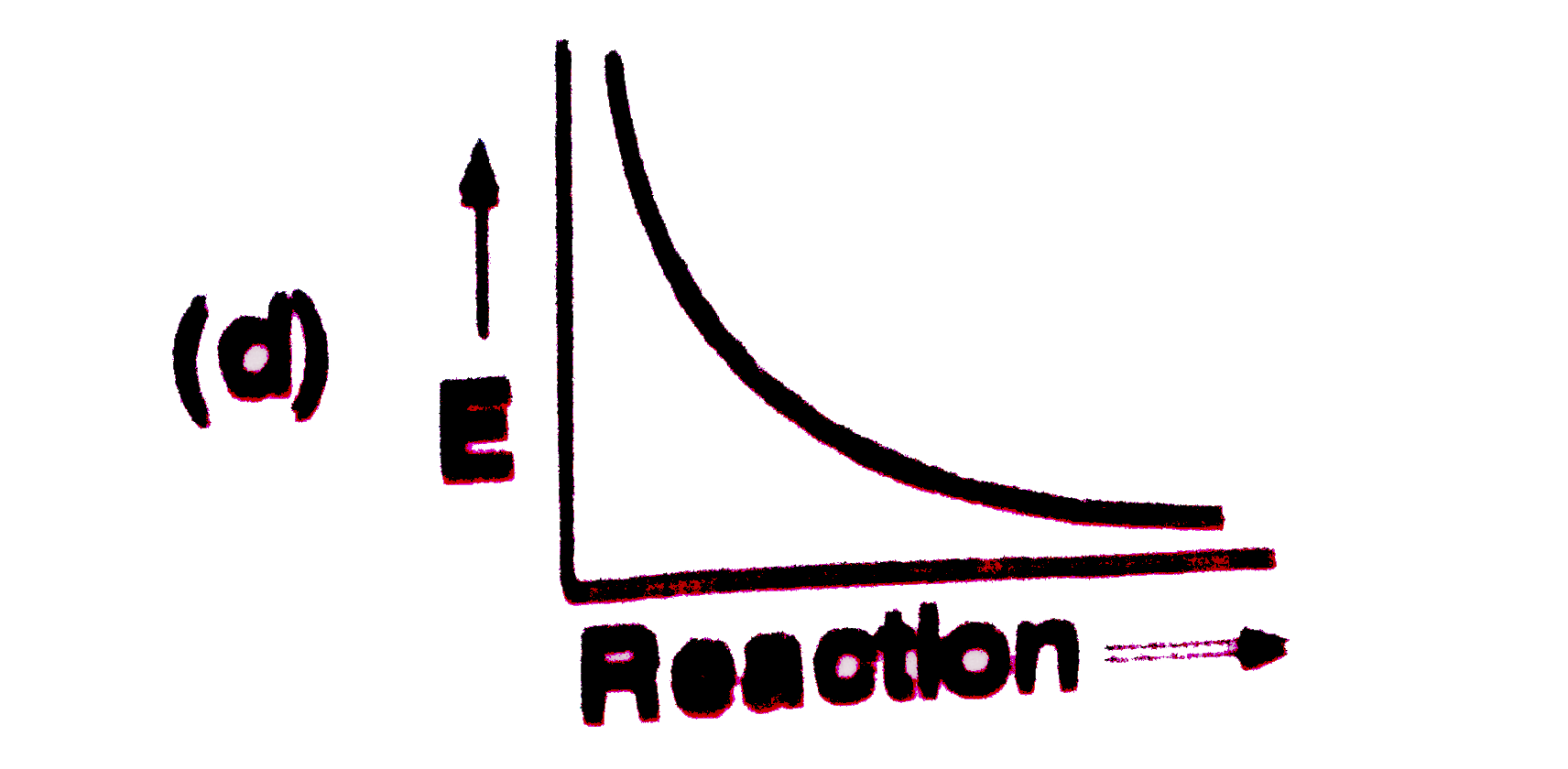
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET I|48 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET II|13 VideosCHEMICAL KINETICS
OP TANDON|Exercise PRACTICE PROBLEMS|70 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-OBJECTIVE QUESTIONS (LEVEL-A)
- Which of the following graphs is correct for second order reaction ?
Text Solution
|
- For the reaction A+B rarr C+D The variation of the concentration of th...
Text Solution
|
- Which graph shows zero activation energy ?
Text Solution
|
- E("Threshold") can never be:
Text Solution
|
- A reaction takes place in theee steps: the rate constant are k(1), k(2...
Text Solution
|
- For hypothetical reaction ArarrB takes placed according to Aoverset(...
Text Solution
|
- If concentration of reactant is increased by 'm' then k becomes :
Text Solution
|
- aB+bBrarrP,dxx//dt=k[A]^(a)8[B]^(b). If conc. of A is doubled, rate is...
Text Solution
|
- A drop of solution (volume 0.05 mL) contains 3 xx 10^(-6) "mole" H^(o+...
Text Solution
|
- For the reaction, H(2)+I(2)overset(k(1))underset(k(2))hArr2HI. The rat...
Text Solution
|
- For the reaction NH(4)^(+)+OCN^(-)rarrNH(2)CONH(2), rthe probable mech...
Text Solution
|
- For a Ist order decomposition of A as given Therefore rate const...
Text Solution
|
- For a reaction A+3BrarrP, Rate = (-d[A])/(dt) ,the expression for the ...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- For Xrarrk=10^(10)e^(-500//T), and for WrarrZ,k=10^(12)e^(-1000//T) at...
Text Solution
|
- For a reversible reaction where the forward reaction is exotherminc, w...
Text Solution
|
- For the reaction , A+3Brarr2C+d which one of the following is not co...
Text Solution
|
- Units of rate constant of first and zero order reactions in terms of m...
Text Solution
|
- Following is the graph between log T(50) and log a (a = initial concen...
Text Solution
|
- Following is the graph between (a-x)^(-1) and time t for second order ...
Text Solution
|