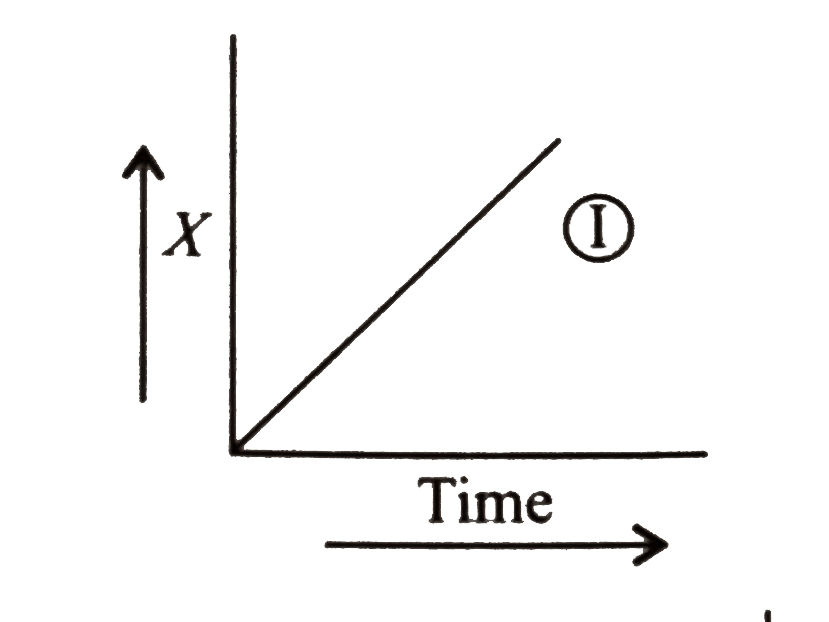
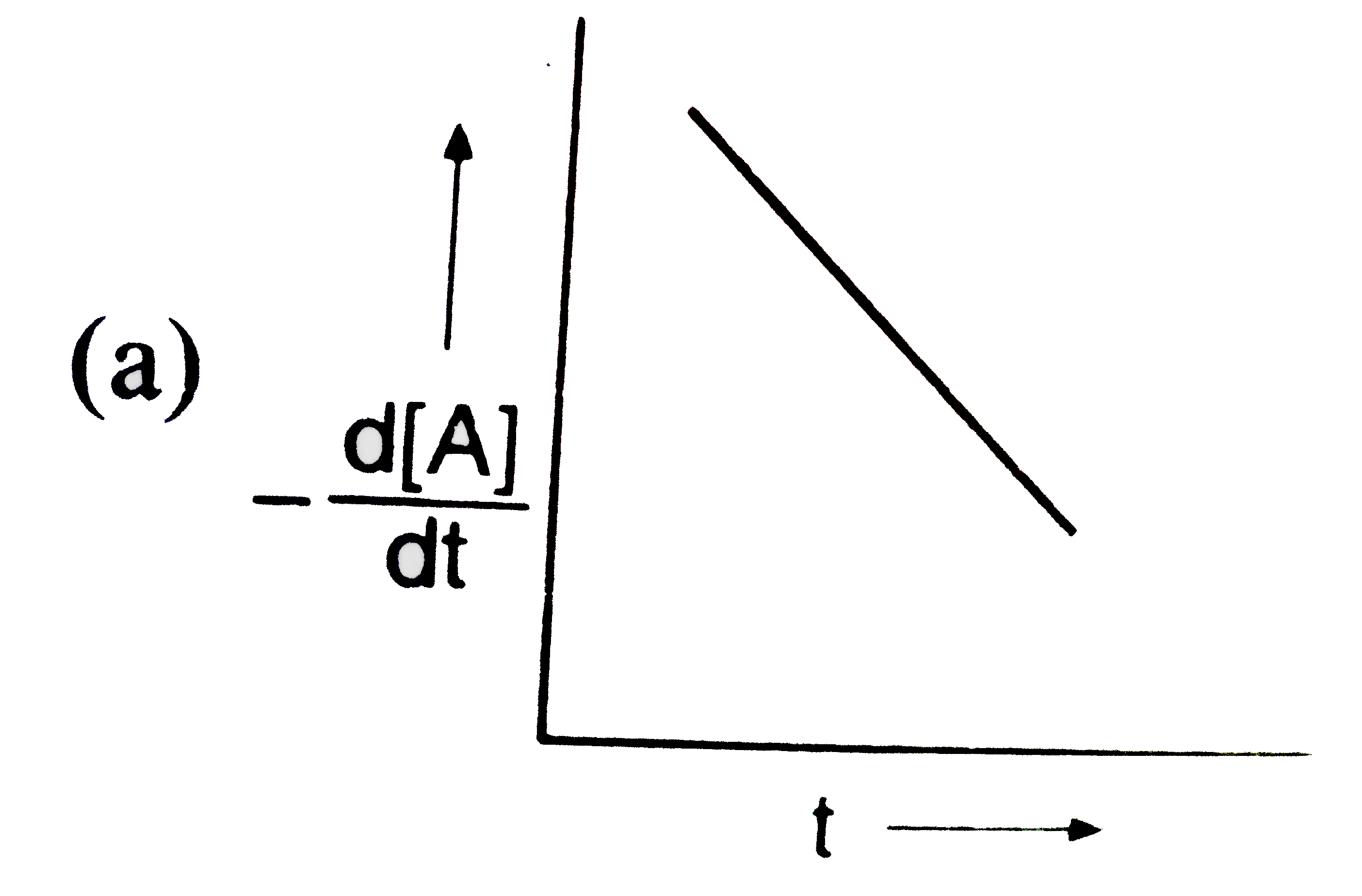
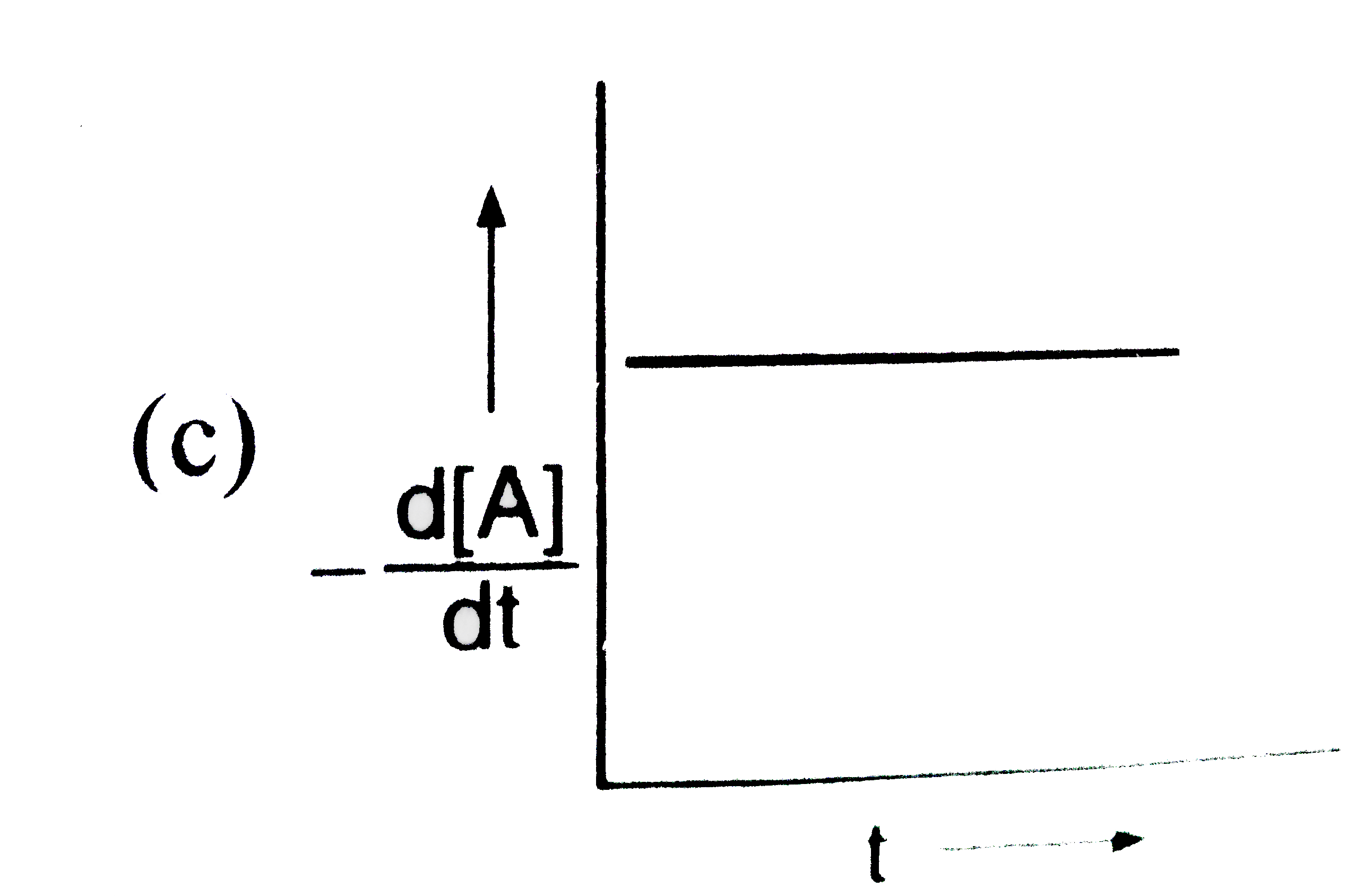
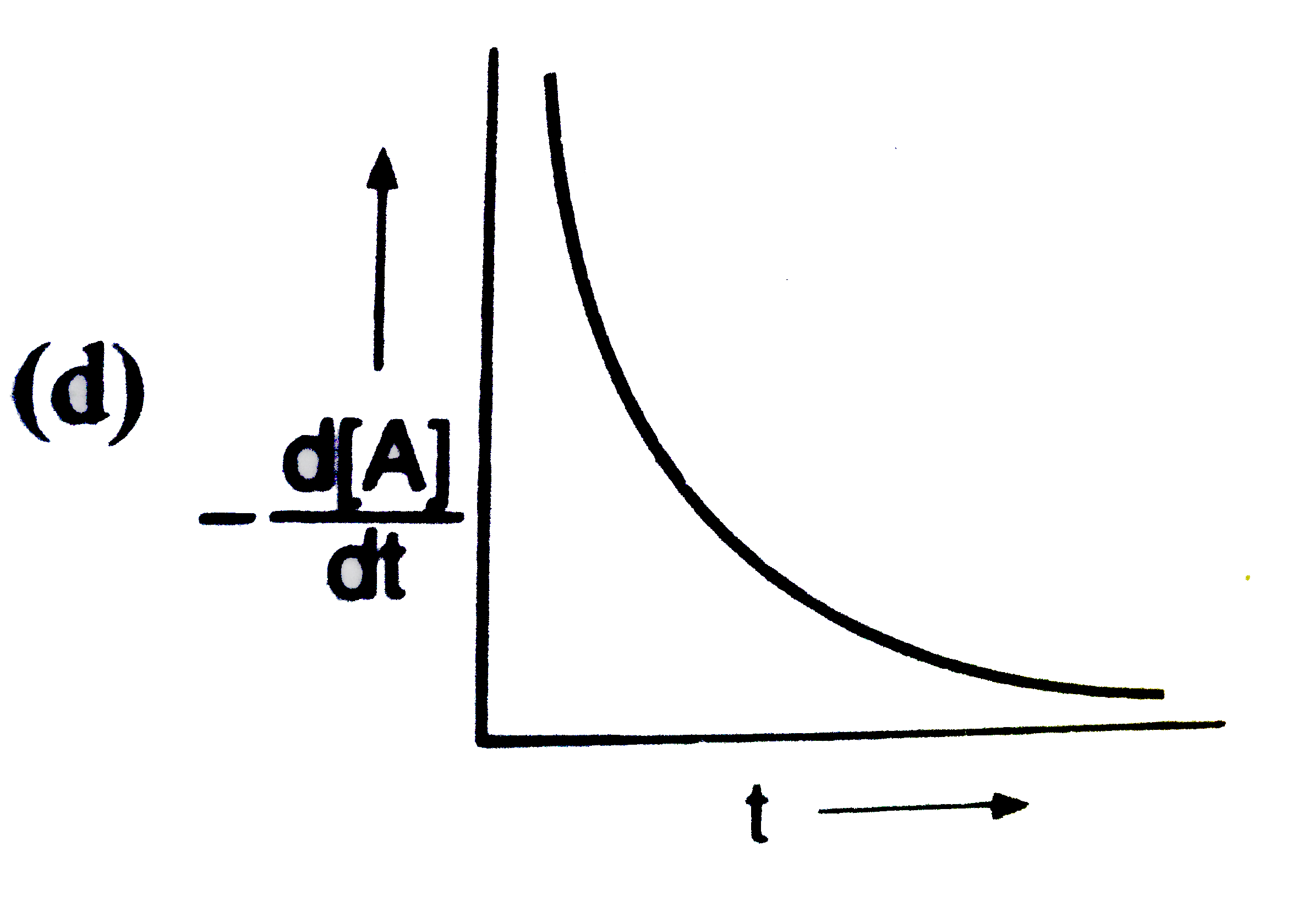
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET I|48 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET II|13 VideosCHEMICAL KINETICS
OP TANDON|Exercise PRACTICE PROBLEMS|70 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-OBJECTIVE QUESTIONS (LEVEL-A)
- Following is the graph between log T(50) and log a (a = initial concen...
Text Solution
|
- Following is the graph between (a-x)^(-1) and time t for second order ...
Text Solution
|
- The graph between concentration (X) of the Product and time of the rea...
Text Solution
|
- Conside the chemical reaction, N(2)(g) + 3H(2)(g)rarr2NH(3)(g) The...
Text Solution
|
- Temerature dependent equation can be written as :
Text Solution
|
- If the rate of reaction ArarrB doubles on increasing the concentratio...
Text Solution
|
- For the reaction : 2N(2)O(5)rarr4NO(g)+O(2)(g) if the concentration of...
Text Solution
|
- A first order reaction is 10% complete in 20 min. the time taken for 1...
Text Solution
|
- An endothermic reaction with high activation energy for the forward re...
Text Solution
|
- For reaction aArarrxP when [A] =2.2 m M, the rate was found to be 2.4 ...
Text Solution
|
- Arrehnius equation for a reaction is given by k=24xx10^(14)e^(-25000//...
Text Solution
|
- Consider the endothermic reaction XrarrY with the activation energies...
Text Solution
|
- Which one the following statement for order of reactions is not correc...
Text Solution
|
- The rate constant of a reaction is found to be 3xx10^(-3)"mol"L^(-1)"m...
Text Solution
|
- In a first order reaction, the concentration of the reactants is reduc...
Text Solution
|
- A substance reacts with initial concentration of a mol dm^(-3) accrodi...
Text Solution
|
- Rate of a reaction can be expressed by Arrhenius equation as: k = Ae...
Text Solution
|
- The rate constant of a first order reaction at 27^(@)C "is" 10^(-3)"mi...
Text Solution
|
- A chemical reaction involves two reacting species. The rate of reactio...
Text Solution
|
- Accroding to Arrhenius equation, the rate constnat (k) is related to t...
Text Solution
|