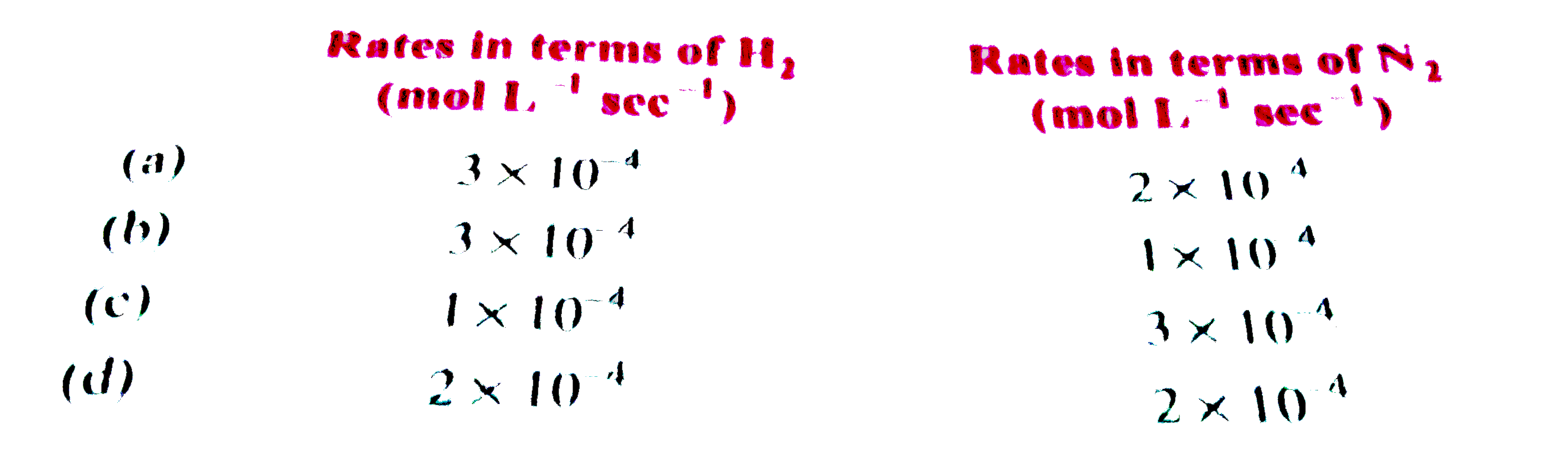Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-B) SET II|13 VideosCHEMICAL KINETICS
OP TANDON|Exercise ASSERTION- REASON TYPE QUESTIONS|14 VideosCHEMICAL KINETICS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-A)|192 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-OBJECTIVE QUESTIONS (LEVEL-B) SET I
- Two different first order reactions have rate consants k(1) and k(2) "...
Text Solution
|
- In the reaction A+BrarrC+D, the rate ((dx)/(dt)) when plotted against ...
Text Solution
|
- In the hober's process of ammonia manufacture, N(2)(g)+3H(2)(g) rarr...
Text Solution
|
- A reaction ArarrB , involes following mechanism : Step1 : Aoverset(...
Text Solution
|
- For a gaseous reaction, the following data were recorded : The or...
Text Solution
|
- The half of second order reactin is :
Text Solution
|
- What names apply to chemical species corresponding to locations 1 and ...
Text Solution
|
- Consider this reaction : 2NO(2)(g)+O(3)(g)rarrN(2)O(5)(g)+O(2)(g) ...
Text Solution
|
- Use the experimental data in the table to determine was studies ...
Text Solution
|
- In which of the following reactions, the increase in the rate of react...
Text Solution
|
- Which of the reactions represented in these diagrams will show the gre...
Text Solution
|
- Which function of [X] . Polleted against time, will give a straight li...
Text Solution
|
- Decomposition of H(2)O(2), is a first order reaction. A 16 volume solu...
Text Solution
|
- What is the activation energy for the reverse of this reaction? N(2)...
Text Solution
|
- The reaction between chloroform, CHCl(3)(g) and chlorine Cl(2)(g) to f...
Text Solution
|
- In the reaction, 3BrO^(-)rarrBrO(3)^(-)+2Br^(-) (aqueous alkaline m...
Text Solution
|
- Which of the following graphs is correct for the following reaction? ...
Text Solution
|
- The activation energy of a certain reaction is 87 kJ "mol"^(-1). What...
Text Solution
|
- Consider the reaction : 2H(2)(g)+2NO(g)rarrN(2)(g)+2H(2)(g) The r...
Text Solution
|
- Propane reacts with iodine in acid medium accroding to the following e...
Text Solution
|