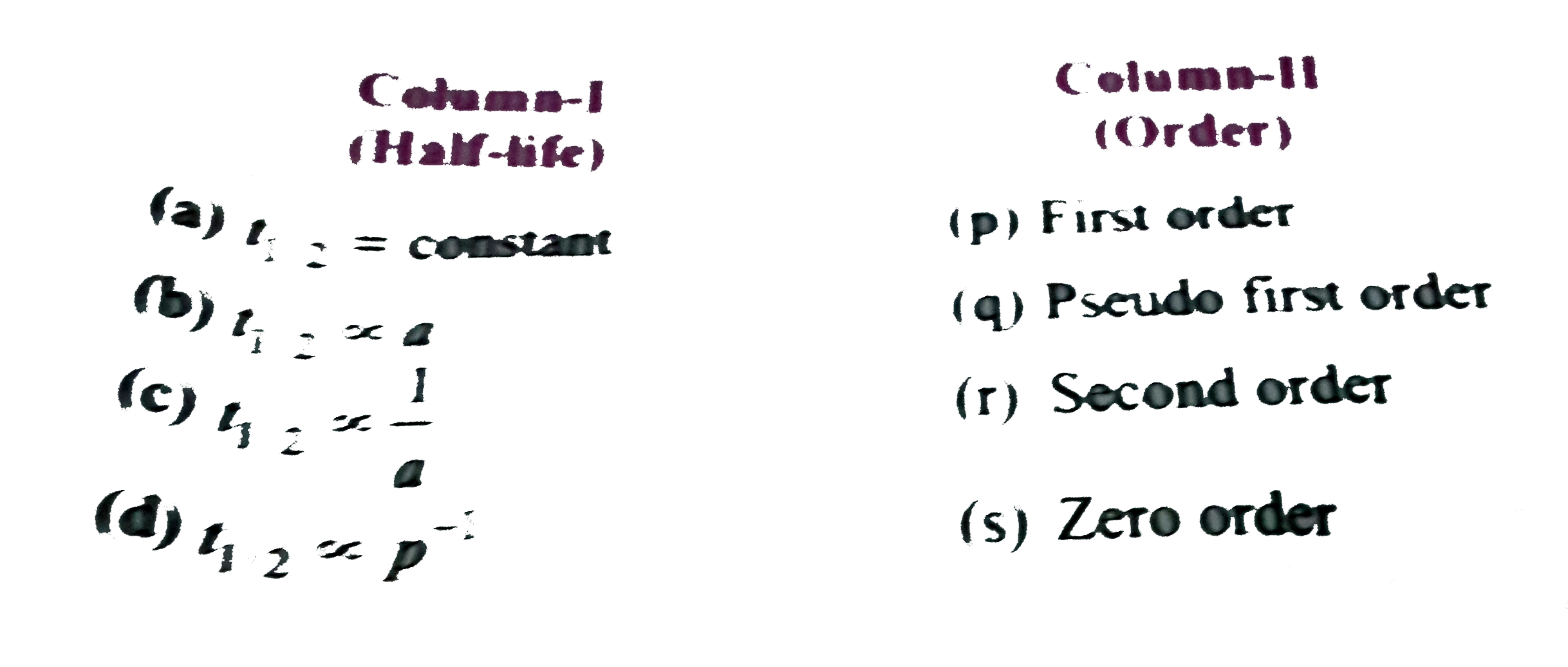Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise INTEGER ANSWER TYPE QUESTIONS|11 VideosCHEMICAL KINETICS
OP TANDON|Exercise PASSAGE -1|6 VideosCHEMICAL KINETICS
OP TANDON|Exercise ASSERTION- REASON TYPE QUESTIONS|14 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-MATRIX MATCHING TYPE QUESTIONS
- Match the reactions of List-I with their ordes in List-II:
Text Solution
|
- Match the reaction in Column-I with the units of their rate constant i...
Text Solution
|
- Match the kinetic equtions of Column-I with the units of their rate co...
Text Solution
|
- Match the half-life in Column-I with the orders in Column-II Whe...
Text Solution
|
- Match the reaction of List-I with the increases in rate when concentra...
Text Solution
|
- Match the List-I with List-II and List-III:
Text Solution
|
- Match the List-I with List-II :
Text Solution
|
- Match the Column-I with Column-II:
Text Solution
|
- Match the Column-I with Column-II:
Text Solution
|
- Match the Column-I with Column-II : Here, t(1//2) = half life t...
Text Solution
|
- Match the Column-I with Column-II :
Text Solution
|