Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise PASSAGE -1|6 VideosCHEMICAL KINETICS
OP TANDON|Exercise PASSAGE -2|4 VideosCHEMICAL KINETICS
OP TANDON|Exercise MATRIX MATCHING TYPE QUESTIONS|11 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CHEMICAL KINETICS-INTEGER ANSWER TYPE QUESTIONS
- In the reaction ArarrB when the initial concentration of reactant is h...
Text Solution
|
- Rate of a chemical reaction increases by 1024 times by 100^(@)C rise i...
Text Solution
|
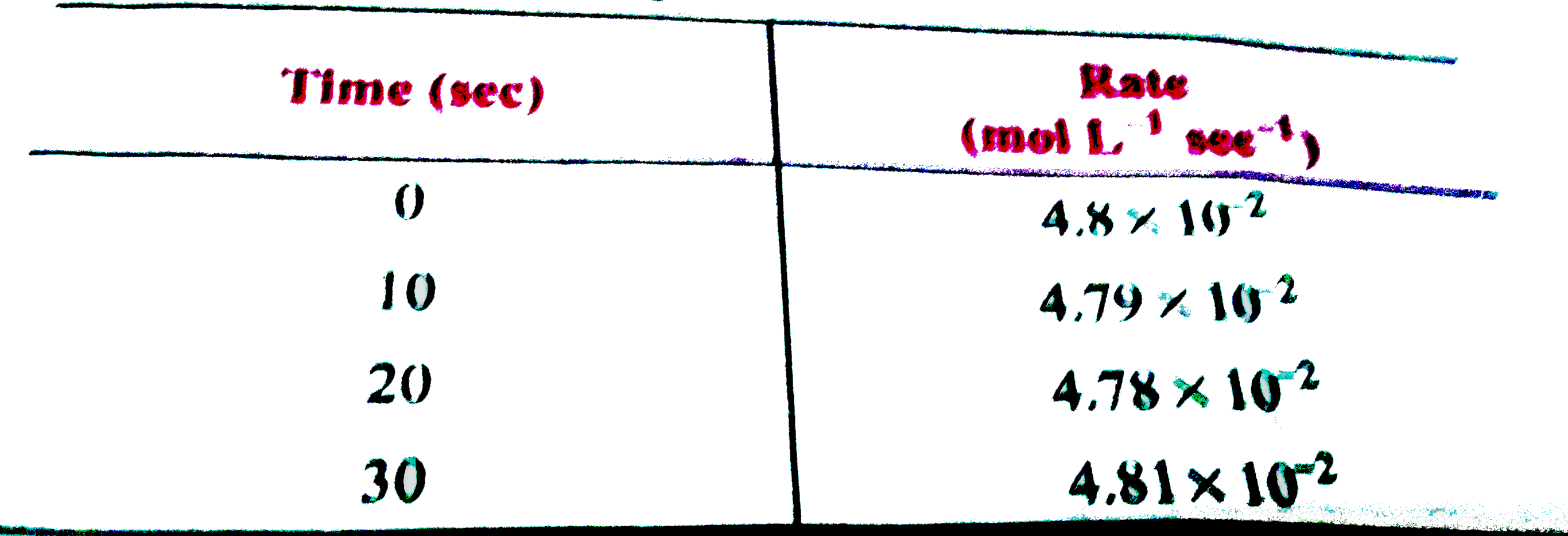
- The rate of a reaction at 10 sec interval are as follows: What wi...
Text Solution
|
- How many times of the half-life will require to complete 75% of a reac...
Text Solution
|
- Ozone deplection takes place as : 2O(3)(g)rarr3O(2)(g) Step1: O(3)...
Text Solution
|
- IF the t(1//2) for a first order reaction 0.4 min, the time of or 99.9...
Text Solution
|
- Consider the following parallel first order reactions The half-li...
Text Solution
|
- The half-life period or a first order reaction is 1 hrs. What is the ...
Text Solution
|
- The half life for a given reaction was doubled as the initial concentr...
Text Solution
|
- For the reaction A(2),+2BrarrAB, the following data were observed : ...
Text Solution
|
- An organic compound undergoes first decompoistion. The time taken for ...
Text Solution
|