A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
OP TANDON|Exercise PASSAGE -2|4 VideosCHEMICAL KINETICS
OP TANDON|Exercise PASSAGE -3|5 VideosCHEMICAL KINETICS
OP TANDON|Exercise INTEGER ANSWER TYPE QUESTIONS|11 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Integer type).|3 Videos
OP TANDON-CHEMICAL KINETICS-PASSAGE -1
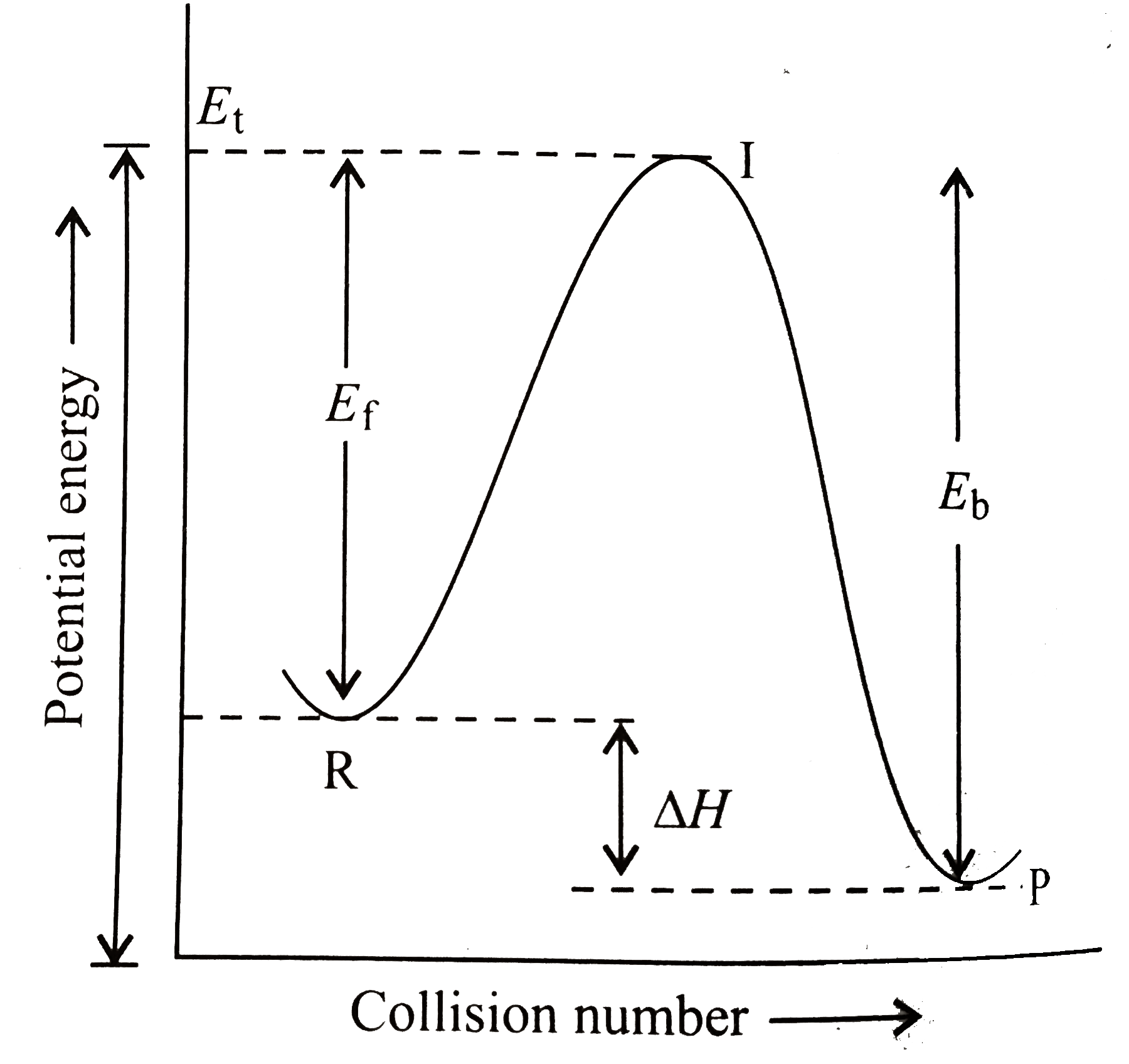
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|