Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
OP TANDON|Exercise Problem For Practice|19 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Matching type|2 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise illustrations of objective questions|10 VideosELECTROCHEMISTRY
OP TANDON|Exercise Matrix-Matching Type Question|3 VideosISOMERISM (STRUCTURAL AND STEROISOMERISM)
OP TANDON|Exercise INTEGER|7 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-HALOALKANES AND HALOARENES -Problem
- How do alkyl, allyl and vinyl halides differ in structure ?
Text Solution
|
- Give the common nad IUPAC names for C(4)H(9)Br isomers and classify th...
Text Solution
|
- (a) Give simple test to distinguish among hexane and CH(3)-CH=CHCI. ...
Text Solution
|
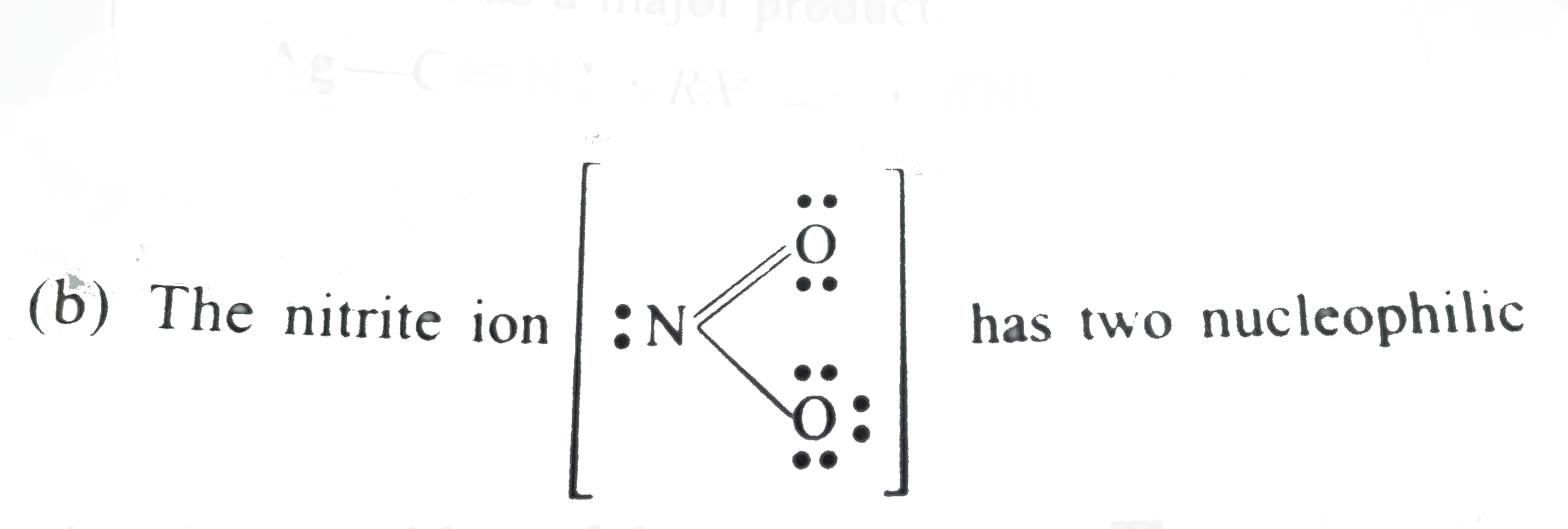
- Give reasons for the following: (a) Potassium cyanide reacts with ...
Text Solution
|
- A halide, C(5)H(11)X, on treating with alc. KOH gives only pent -2- en...
Text Solution
|
- A white precipitate was formed slowly when silver nitrate was added to...
Text Solution
|
- How many isomers are possible for C(4)H(8)F(2) and give their IUPAC na...
Text Solution
|
- Give the structures of two different alkyl bromides both of which yiel...
Text Solution
|
- (a) Indicate whether the following are S(N^(1)),S(N^(2)),E(1) " or "E...
Text Solution
|
- (a) Which has faster rate of S(N^(1))? (b) Which has faster rate ...
Text Solution
|
- Draw the products of the following reaction with organometallic reagen...
Text Solution
|
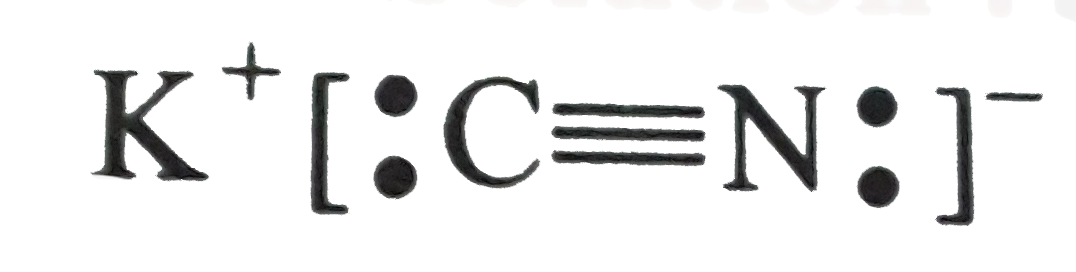 and provide cyanide ions in solution, in which each of carbon and nitrogen carry a lone pair of elctrons [CN is an ambident nucleophile(ligand)].
and provide cyanide ions in solution, in which each of carbon and nitrogen carry a lone pair of elctrons [CN is an ambident nucleophile(ligand)].