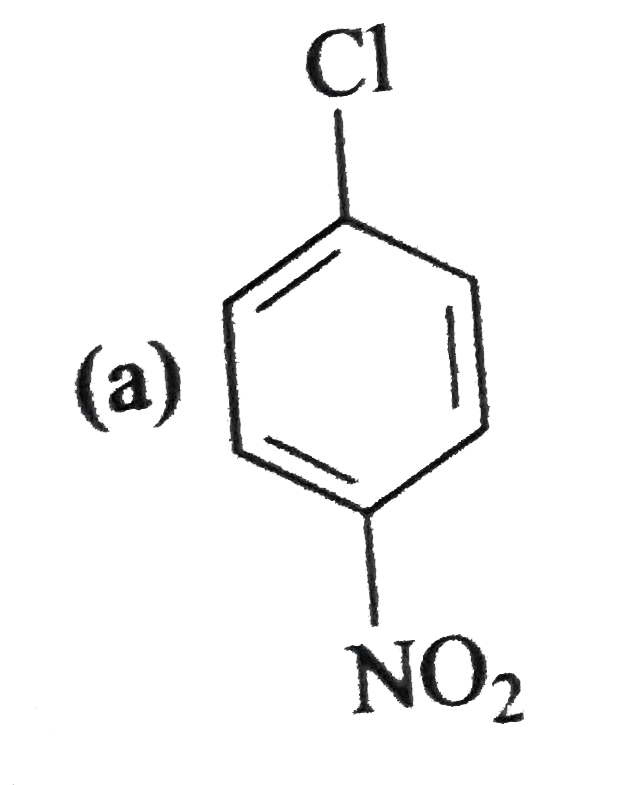
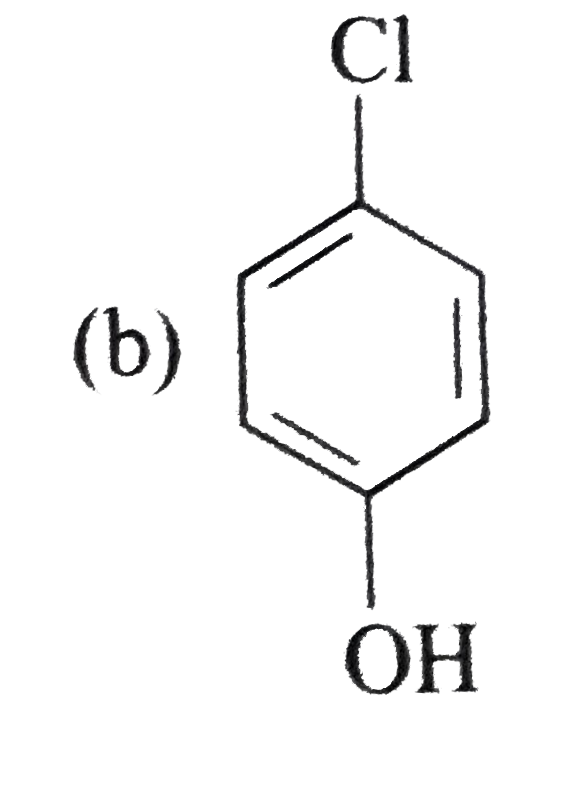
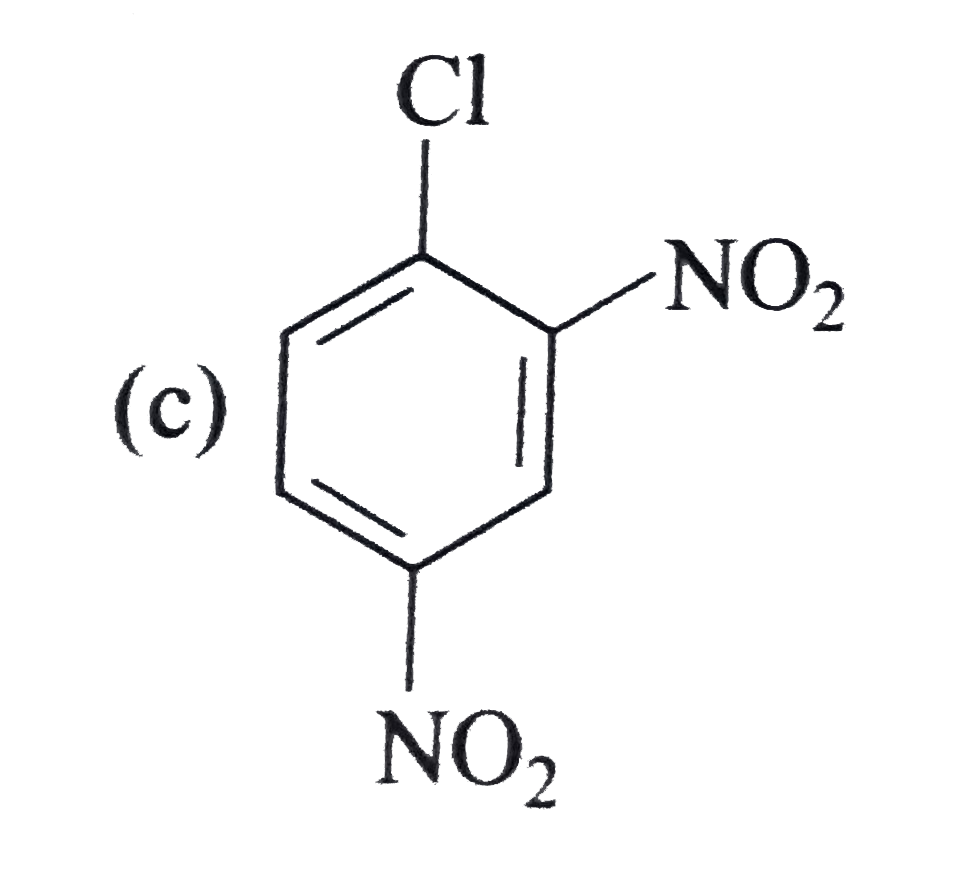
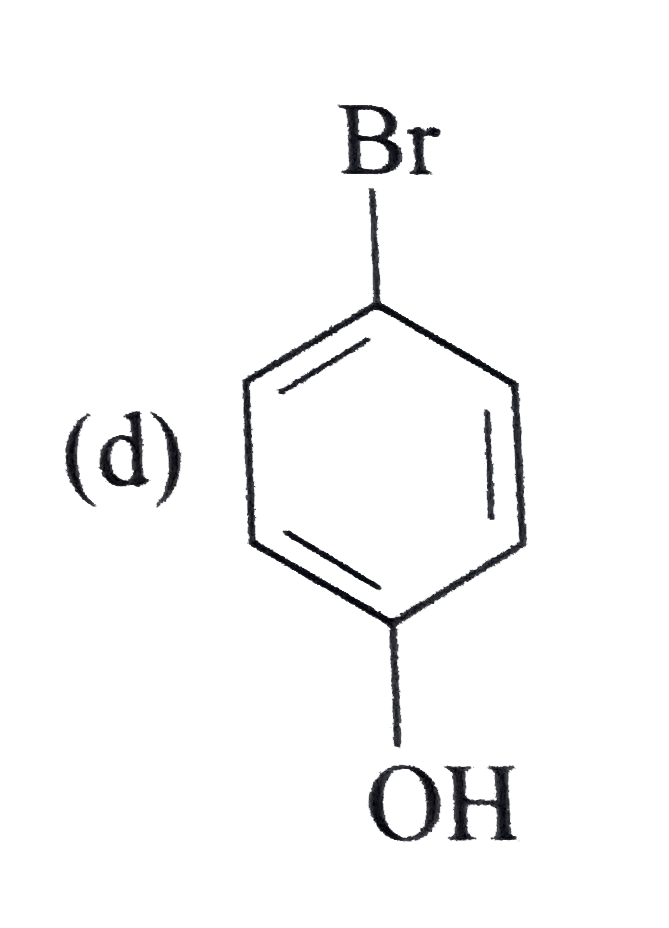
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
OP TANDON|Exercise Objective Questions Lavel -B Set II This set contains the questions|16 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Assertion -Reason Type Questions|20 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Objective Questions|234 VideosELECTROCHEMISTRY
OP TANDON|Exercise Matrix-Matching Type Question|3 VideosISOMERISM (STRUCTURAL AND STEROISOMERISM)
OP TANDON|Exercise INTEGER|7 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-HALOALKANES AND HALOARENES -Objective Questions Lavel -B
- Dehydrohalogenation by strong base is slowest in :
Text Solution
|
- consider the structures of the following two molecules : X : F(2)C=C...
Text Solution
|
- Which of the following is least reactive towards nucleophilic substit...
Text Solution
|
- water (through S(N^(2)) reaction mechanism) then sterochemistry of pro...
Text Solution
|
- Which of the following is known as freon which is used as a refrigeran...
Text Solution
|
- In which of the following reactions an otically active single product ...
Text Solution
|
- Which of the following will give acetophenone as a product ?
Text Solution
|
- Most reactive alkyl halide towards E2 mechanism is :
Text Solution
|
- Which of the following compounds is the most likeyl to undergo a bimo...
Text Solution
|
- The major product obtained in the reaction
Text Solution
|
- The reaction H(2)C=CH-CH(3)+CI(2) overset(673K)(to) H(2)C=CH-CH(2)C...
Text Solution
|
- Iodoform si used as an:
Text Solution
|
- The trade name of trichloroethylene is :
Text Solution
|
- In the reaction : R-Br+CI^(-) to R -CI+Br^(-) The rates of react...
Text Solution
|
- Arrange the following compounds in the decreasing order of the boiling...
Text Solution
|
- In the reaction , CH(3)-underset(Br)underset(|)(CH)-CH(3)overset(alc...
Text Solution
|
- C Cl4 is used as fire extinguisher because :
Text Solution
|
- The correct of reactivity of alkyl halides for S(N^(1)) reaction is :
Text Solution
|
- Match List I with List II and select the correct answer using the co...
Text Solution
|
- Match List I with list II and select the correct answer using the cod...
Text Solution
|