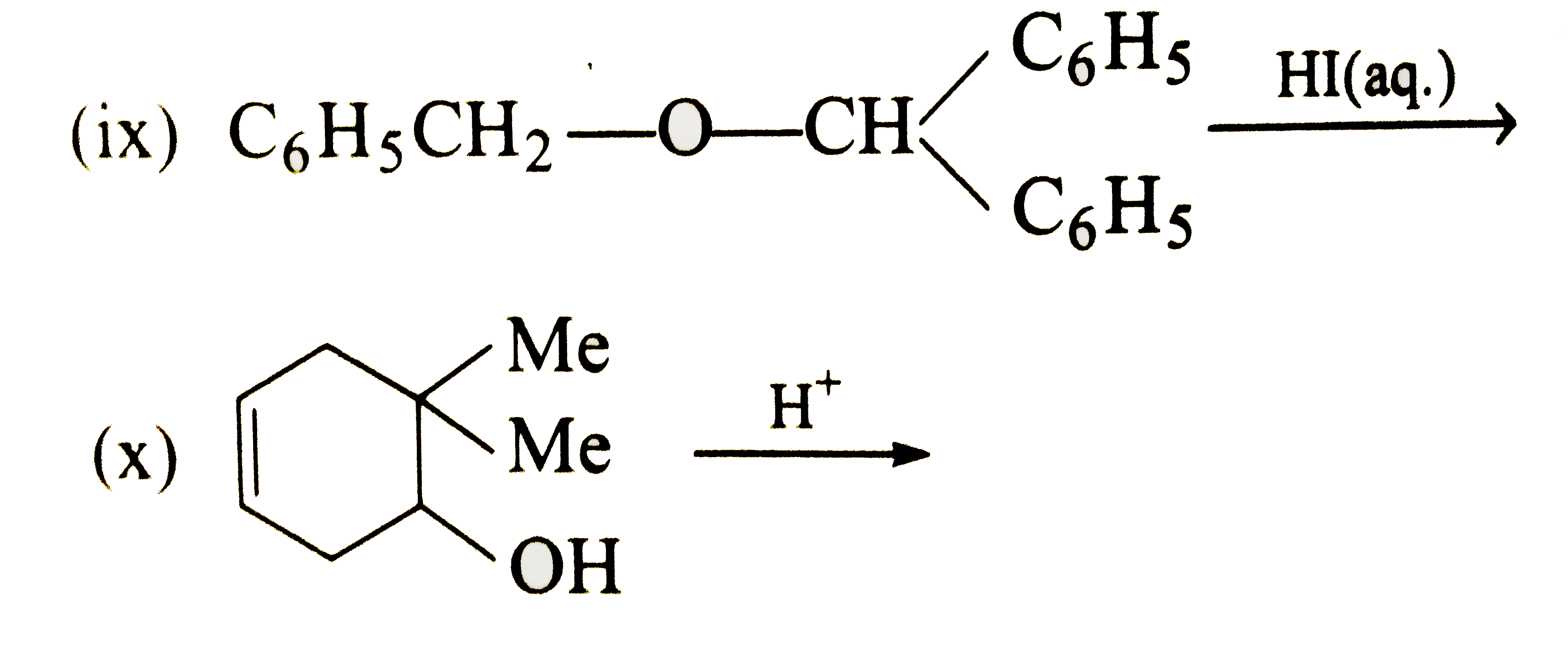
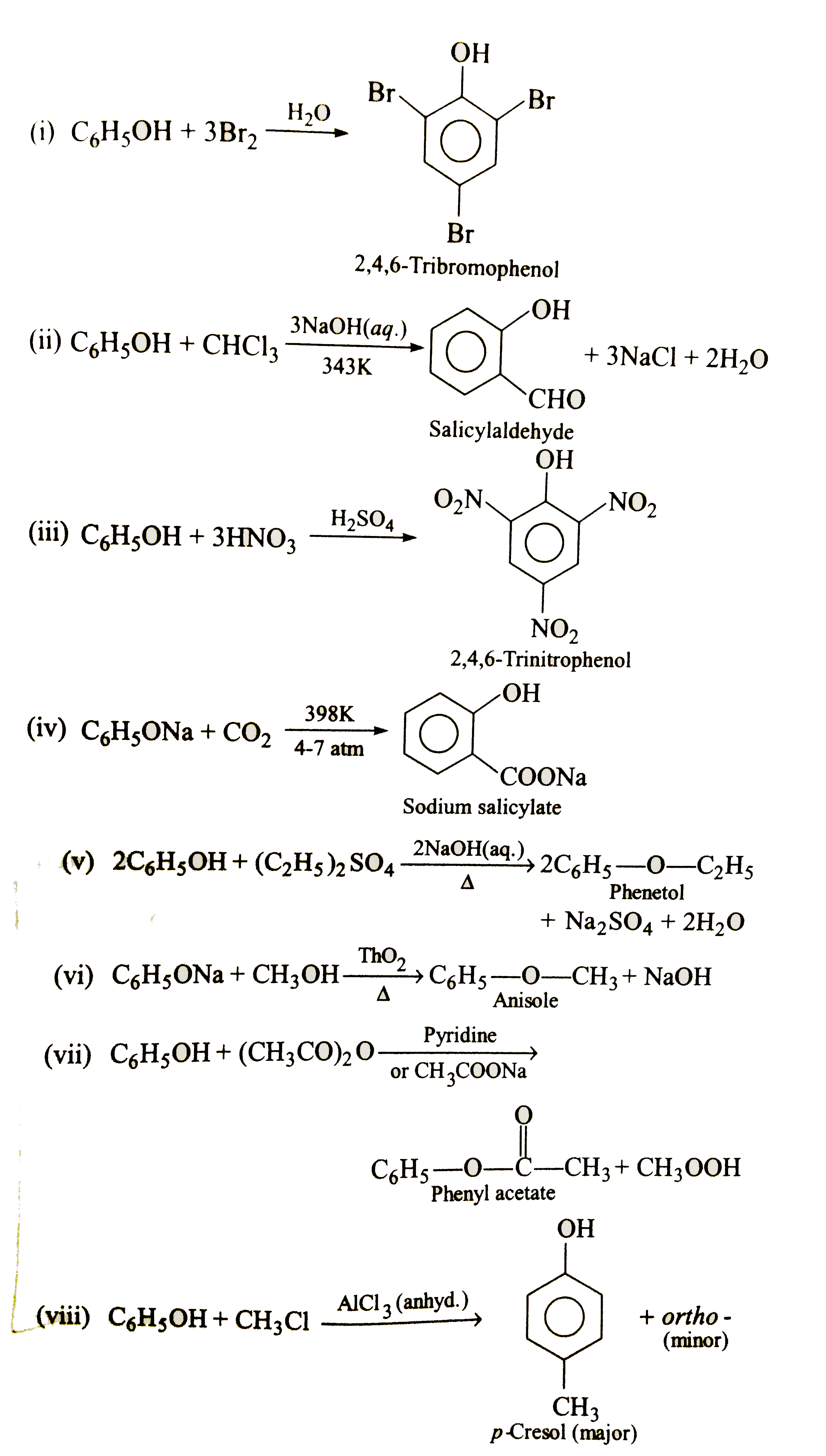
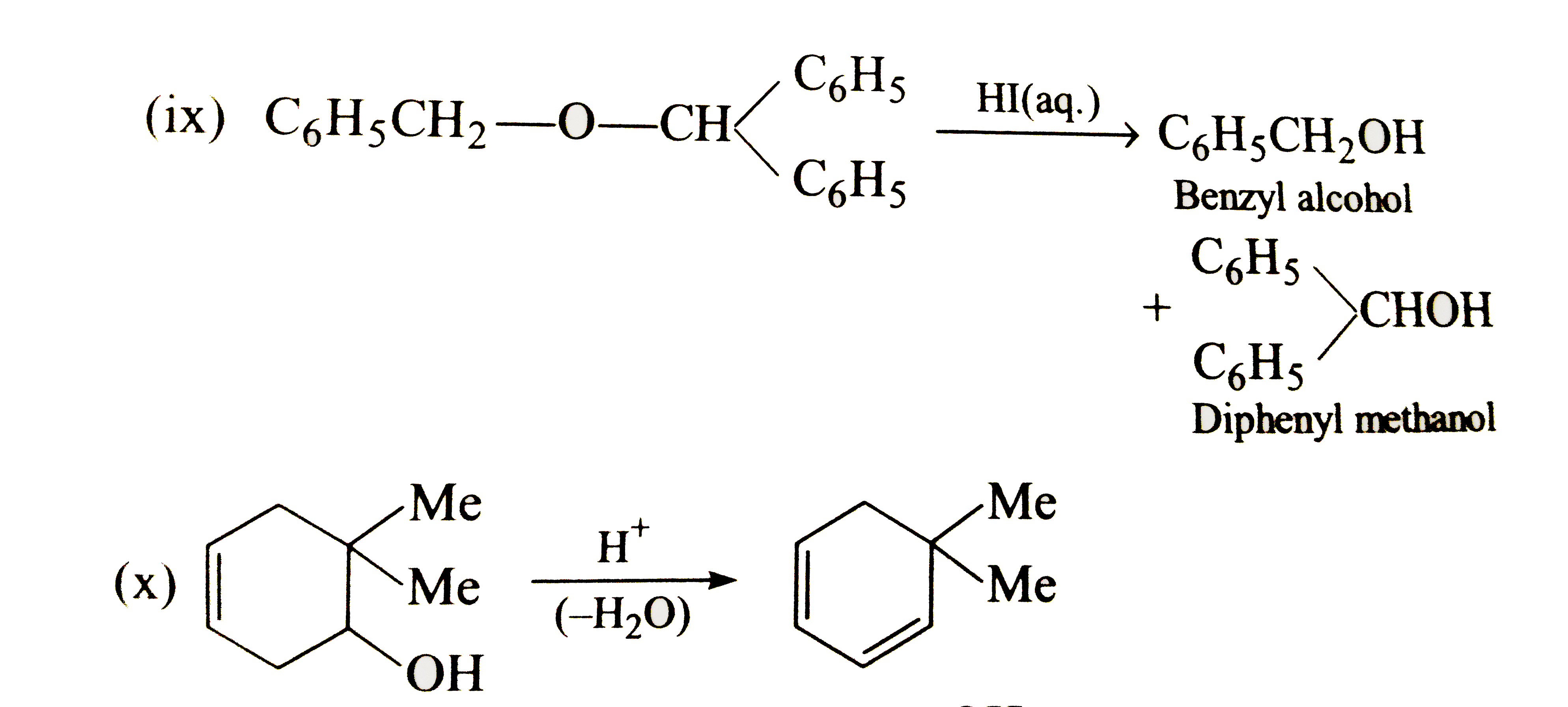
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise ETHERS|33 VideosALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise PROBLEMS BASED ON STRUCTURE AND PROPERTIES|7 VideosALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise Matching Type|2 VideosALDEHYDES AND KETONES
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ALCOHOLS, PHENOLS AND ETHERS-PHENOLS
- Complete the following equations : (i) C(6)H(5)OH+Br(2)overset(H(2)O...
Text Solution
|
- Identify the final product in each reaction.
Text Solution
|
- Identify the unknown compounds
Text Solution
|
- Arrange the following : (a) C(6)H(5)OH,m-ClC(6)H(4)OH and p-ClC(6)H...
Text Solution
|
- Answer the following : (i) Phenol is more acidic than ethyl alcohol....
Text Solution
|
- How the following pairs can be distinguished ? (i) Phenol and benzoi...
Text Solution
|
- What happens when ? (i) Cumene hydroperocide is treated with dilute ...
Text Solution
|
- How will you convert phenol into ? 9i) Salicylaldehyde (Reimer-Tiema...
Text Solution
|
- Describe the following : (i) Reimer -Tiemann reaction (ii) Kolbe's...
Text Solution
|
- Carbolic acid is
Text Solution
|
- Benzyl alcohol and phenol can be distinguished by using
Text Solution
|
- Which of the following reagents may be used to distinguish between phe...
Text Solution
|
- When sodium benzene sulphonate is fused with sodium hydroxide (solide)...
Text Solution
|
- When phenol is treated with excess of bromine water, it gives
Text Solution
|
- Picric acid is a yellow coloured compound. Its chemical name is
Text Solution
|
- Sodium phenoxide when heated with CO(2) under pressure at 398K gives :
Text Solution
|
- Phenol when condensed with phthalic anhydride in the presence of conc....
Text Solution
|
- Phenol is heated with C Cl(4) and alkaline KOH when salicylic acid is ...
Text Solution
|
- Phenol reacts with bromine in carbon disulphide at low temperature to ...
Text Solution
|
- Of the two compounds shown below , the vapour pressure of B at a par...
Text Solution
|