A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
OP TANDON-ALCOHOLS, PHENOLS AND ETHERS-Passage 4
- Carbon-oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon-oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon-oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon-oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon-oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
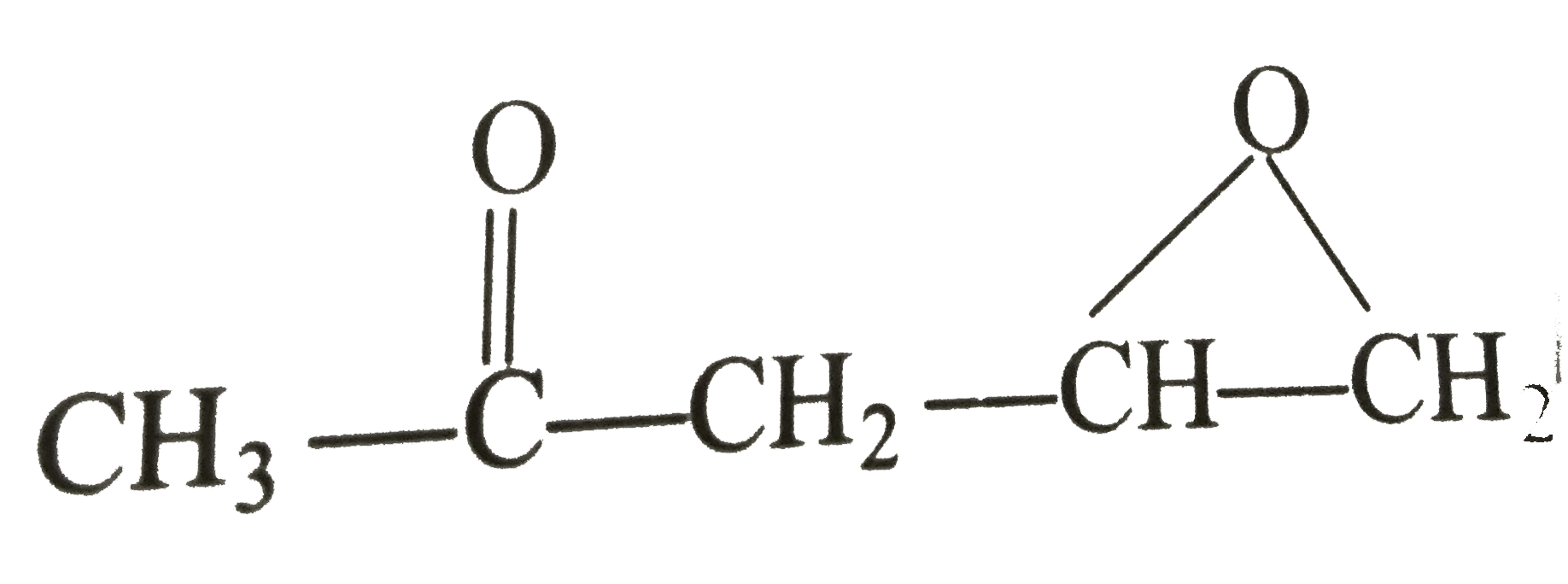 `underset(D_(2)O)overset(LiAlH_(4))(rarr)'Y' , ` Identify 'Y'`:`
`underset(D_(2)O)overset(LiAlH_(4))(rarr)'Y' , ` Identify 'Y'`:`