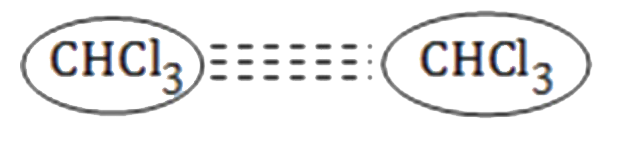
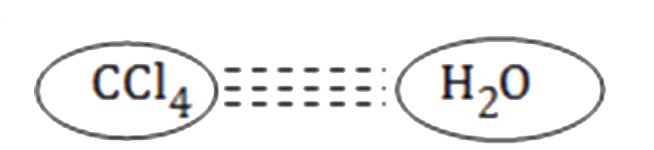
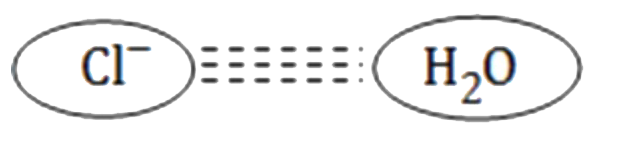
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 104-CHEMISTRY
- How long a current of 3 ampere has to be passed through a solution of ...
Text Solution
|
- In which of the following molecular species both sigma-dative and pi- ...
Text Solution
|
- Which of the following attraction is strongest?
Text Solution
|
- A metal gets coated with a green basic carbonate when exposed to atmos...
Text Solution
|
- Dissolution of a solute is an exothermic process if
Text Solution
|
- In the given reaction CH(3)-CHO overset(2HCHO //dil.Na2CO3)(rarr)(X)...
Text Solution
|
- In the given reaction 'X' will be
Text Solution
|
- Calculate the heat required to make 6.4kg of CaC2 from CaO(s) and C(s)...
Text Solution
|
- In chemical reaction of potassium ferrocyanide and hydrogen peroxide i...
Text Solution
|
- Red hot carbon will remove oxygen from the oxides XO and Yo but not fr...
Text Solution
|
- Calcium lactate is a salt of weak acid and represented as Ca(LaC)(2). ...
Text Solution
|
- Which of these compounds will not form tribromo derivative ?
Text Solution
|
- The reagent( s) used in the preparation of aspirin from salicylic acid...
Text Solution
|
- Lyophilic sols are more stable than lyophobic sols because :
Text Solution
|
- The rate constant K(1) of a reaction is found to be double that of rat...
Text Solution
|
- Exess of KI reacts with CuSO(4) solution and then Na(2)S(2)O(3) soluti...
Text Solution
|
- When an ideal binary solution is in equilibrium with its vapour, molar...
Text Solution
|
- What is the coordination number of Ni in nickel DMG complex?
Text Solution
|
- Which of the following set of statements regarding the SN1 reaction sh...
Text Solution
|
- 10 ml gaseous hydrocarbon was burnt completely in 80 ml of O(2) at NTP...
Text Solution
|