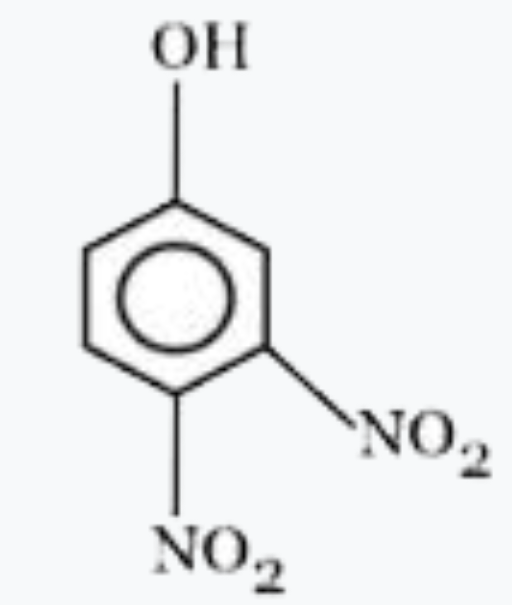
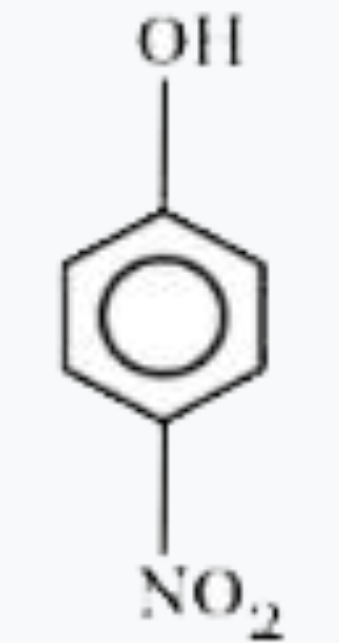
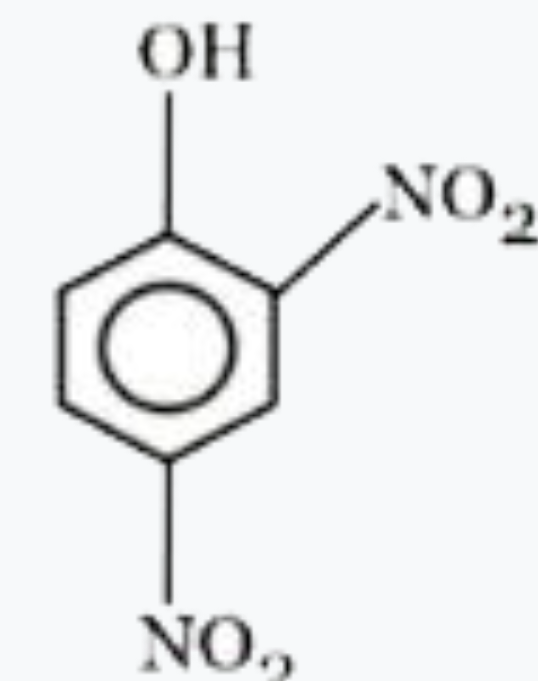
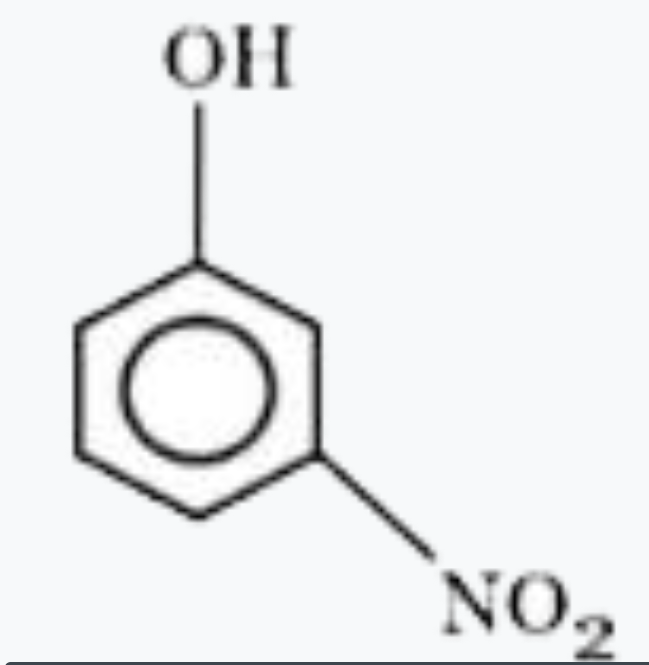
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET TEST 111-CHEMISTRY
- What is the position of the element Z = 20 in the modern periodic tabl...
Text Solution
|
- The correct order of ionization energy in the following second-period ...
Text Solution
|
- Most acidic hydrogen containing compound among the following is
Text Solution
|
- During the test of halogens by silver nitrate test, the sodium extract...
Text Solution
|
- Which of the following is definitely true, if for a reaction activatio...
Text Solution
|
- For three reactions of first, second and third-order, the numerical va...
Text Solution
|
- The correct increasing order of pH of 0.1 M solution of the following ...
Text Solution
|
- Determine the solubility of Cr(OH)(3) in mol L^(-1), If its K(sp) is 2...
Text Solution
|
- If 965 coulombs of electricity is passed through a metal cup dipped in...
Text Solution
|
- The standard electrode potentials (E(M^(+)//M)^(@)) of four metals A, ...
Text Solution
|
- What is the molar conductance (in S cm^(2) mol^(-1)) of 1M solution of...
Text Solution
|
- Find the natural polymer among the following
Text Solution
|
- What does cellulose give on complete hydrolysis?
Text Solution
|
- Find the enthalpy of dissociation of H2C2O4 from the given data: Entha...
Text Solution
|
- In which of the following conditions, a reaction will definitely be sp...
Text Solution
|
- The number of isomers of C6H14 is
Text Solution
|
- Among the given options, this compound will exhibit:
Text Solution
|
- The empirical formula of a mixed oxide. In which the oxide ions are pr...
Text Solution
|
- The suspension milk of lime is composed of:
Text Solution
|
- A metal M readily forms water soluble sulphate, and water insoluble hy...
Text Solution
|