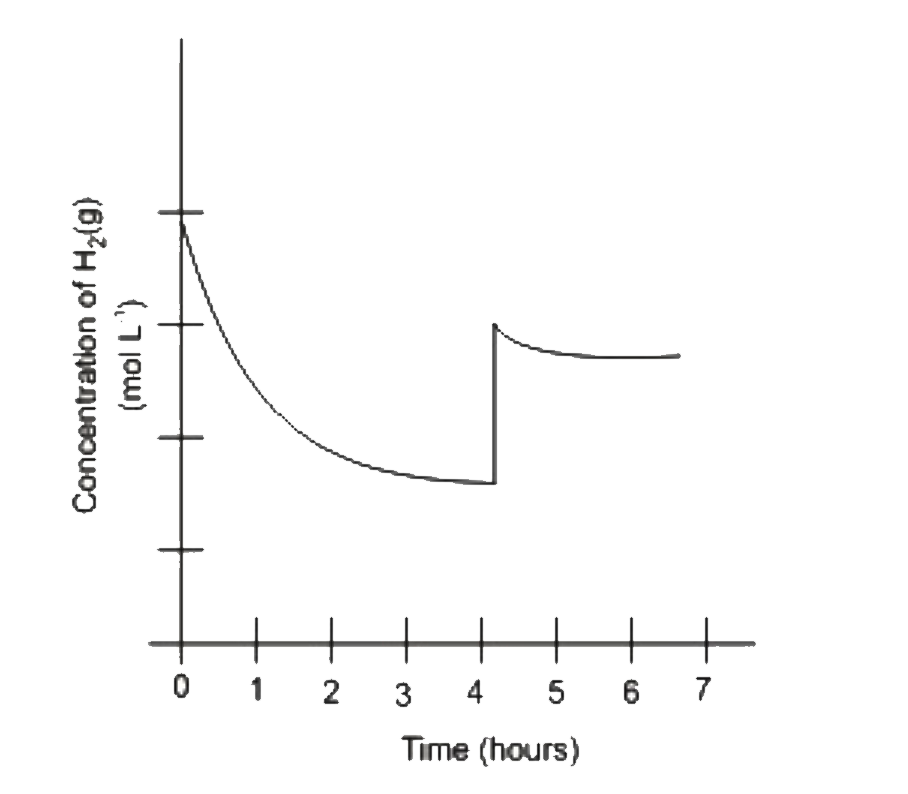
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 107-CHEMISTRY
- The pH of 10^(-8)M solution of HCl in water is
Text Solution
|
- In the synthesis of glycerol from propene, the steps involved are :
Text Solution
|
- In brown ring Fe and NO exist as Fe^(+) and NO^(+) rather than Fe^(2+)...
Text Solution
|
- The percentage of oxygen in CH(2) O is
Text Solution
|
- Which of the followingis not correct?
Text Solution
|
- Which among the following is the most reactive
Text Solution
|
- A compound contains three elements X, Y and Z. The oxidation number of...
Text Solution
|
- Identify the metal M whose extraction is based on the following reacti...
Text Solution
|
- The reaction between phosphorus (P(4)) and hydrogen (H(2)) can result ...
Text Solution
|
- Rate of physisorption increases with :
Text Solution
|
- The nodal plane in the pi-bond of ethene is located in:
Text Solution
|
- The solubility of fluorides of alkali metals in water is
Text Solution
|
- The substance used in the thermite process of reducing metal ores is
Text Solution
|
- In the hydrogen atom spectrum, the least energetic photon takes place ...
Text Solution
|
- What is the activation energy for the decomposition of N(2)O(5) as N...
Text Solution
|
- Number of chiral centres in Pencillin is
Text Solution
|
- Diamonds are formed from graphite under high pressure in coal mines. C...
Text Solution
|
- At 300 K, 36 g of glucose present per litre in its solution has an osm...
Text Solution
|
- In borax number of B-O-B bonds are X and number of boron atoms are Y. ...
Text Solution
|
- Find the molaity of water. Given: rho =1000kg//m^(3) [Report your ...
Text Solution
|