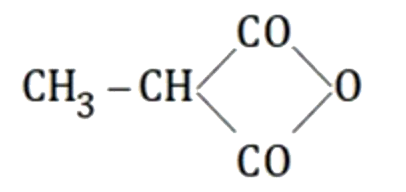
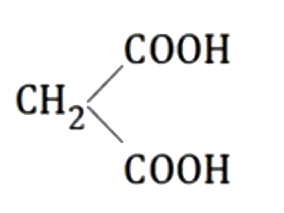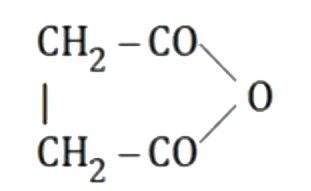A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 108-CHEMISTRY
- The ionization constant of overset(o+)(NH(4)) ion in water is 5.6 xx 1...
Text Solution
|
- Match List I with List II and select the correct anwer using the codes...
Text Solution
|
- When H(2) is added to two isomeric alkenes A and B having molecular fo...
Text Solution
|
- Which of the following set is having correct statements here regarding...
Text Solution
|
- The heat of neutralisation of a strong base and a strong acid is 13.7 ...
Text Solution
|
- Approximate pH of 0.01M aqueous H(2)S solution, when K(1) and K(2) for...
Text Solution
|
- The ionization enthalpies of Li and Na are "520 kJ mol"^(-1) and "495 ...
Text Solution
|
- Which of the following given below can liberate Br(2) from KBr? F(2)...
Text Solution
|
- In the given reaction, OHC-(CH(2))(3)-overset(O)overset(||)C-CH(3)ov...
Text Solution
|
- 5g sulphur is present in 100 g of CS(2). DeltaT(b) of solution 0.954 a...
Text Solution
|
- The halides which can react with water or undergo hydrolysis are PCl(5...
Text Solution
|
- Which one of the following are examples of Sandmeyer reaction?
Text Solution
|
- Identify the final product 'W' in the given reaction sequence
Text Solution
|
- In the given reaction CH(3)-COOH underset(underset("(iii) "H(2)O //H^(...
Text Solution
|
- Arrange the following in increasing order of acidic strength
Text Solution
|
- An organic compound (P) having molecular mass 180 is acylated with CH(...
Text Solution
|
- A sample of H(2) SO(4) (density 1.787 g mL^(-1)) is labelled as 86% by...
Text Solution
|
- The possible numerof structural and stereo isomers for the complex [MB...
Text Solution
|
- An electrolytic cell is constructed for preparing hydrogen. For an ave...
Text Solution
|
- For the equilibrium: LiCl.3NH(3(s))hArrLiCl.NH(3(s))+2NH(3), K(p)=9 ...
Text Solution
|