A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 109-CHEMISTRY
- N(2) and O(2) are converted into monocations, N(2)^(+) and O(2)^(+) r...
Text Solution
|
- Consider the following reactions in which all the reactants and the pr...
Text Solution
|
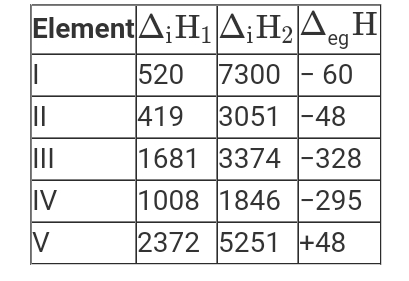
- The first (Delta(i)H(1)) and second (Delta(i)H(2)) ionization enthalpi...
Text Solution
|
- Equal volumes of 0.1 M NaCl and 0.06 M CaCl(2) solutions are separated...
Text Solution
|
- The correct match between Item I and Item II is
Text Solution
|
- The increasing order of the pK(a) value of the following compounds is
Text Solution
|
- AgCl dissolved in excess of NH(3), KCN and Na(2)S(2)O(3) solutions the...
Text Solution
|
- Considered the following statements : 1. Zeolites are aluminosilicat...
Text Solution
|
- Identify the final product 'E' in the given sequence of reactions here
Text Solution
|
- The flocculating power of the given ions for the specified colloidal s...
Text Solution
|
- Among (a) -(d) the complexes that can display geometical isomerism ar...
Text Solution
|
- The correct match between Item 'I' and Item 'II' is
Text Solution
|
- Mark the correct statements(s) (1) Manganeses exhibits +7 oxidation ...
Text Solution
|
- Arrange following compounds in decreasing order of rate of electrophil...
Text Solution
|
- The given plots represent the variation of the concentration of a reac...
Text Solution
|
- Chlorine reacts with hot and concentrated NaOH and produces compounds ...
Text Solution
|
- In the Kjeldahl's method for estimation of nitrogen present in a soil ...
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- The number of stereo isomers possible for a compound of the molecular ...
Text Solution
|
- The percentage of p-character in the orbitals forming p-p bonds in P4 ...
Text Solution
|