Similar Questions
Explore conceptually related problems
Recommended Questions
- With the help of the following equation and data choose the wrong stat...
Text Solution
|
- ArarrB, DeltaH= -10 KJ mol^(-1), E(a(f))=50 KJ mol^(-1), then E(a) of ...
Text Solution
|
- With the help of the following equation and data choose the wrong stat...
Text Solution
|
- For an endothermic reaction energy of activation is E(a) and enthlpy o...
Text Solution
|
- The successive ionization energies (in kJ/mol) for an element are show...
Text Solution
|
- {:(I.,E(a)=15m "kJ mol"^(-1),,DeltaH=-70 "kJ mol"^(-1)),(II.,E(a)=30"k...
Text Solution
|
- For an endothermic reaction , energy of activation is E(a) and enthalp...
Text Solution
|
- Following are the values of E(a) and DeltaH for three reactions carrie...
Text Solution
|
- 2A rarr B+C The mechanism of the above reaction given as 2A overset(K(...
Text Solution
|
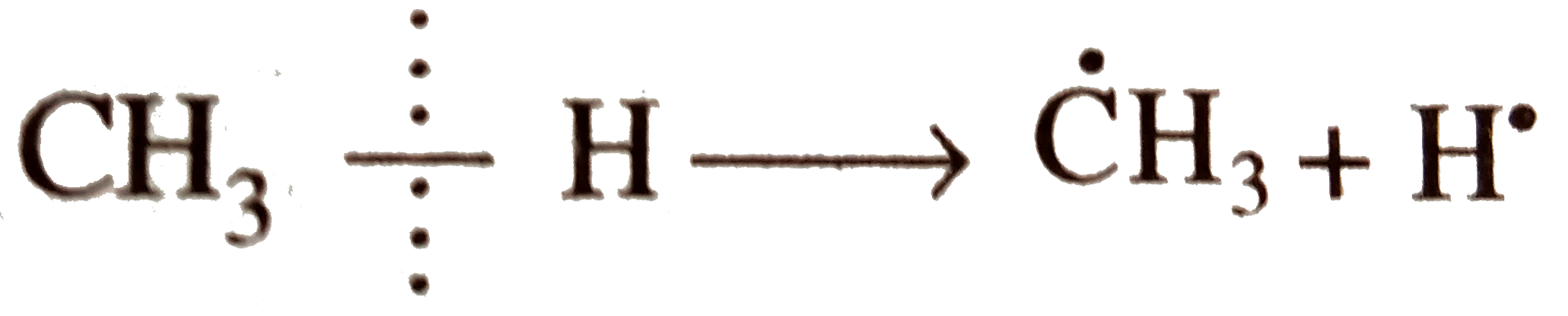 `E_(act) = + 435.1 kJ`
`E_(act) = + 435.1 kJ` 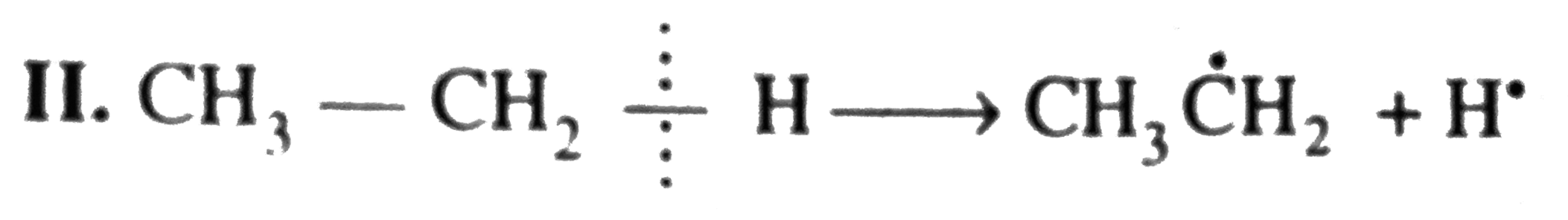 `E_(act) = + 410.0 kJ`
`E_(act) = + 410.0 kJ` 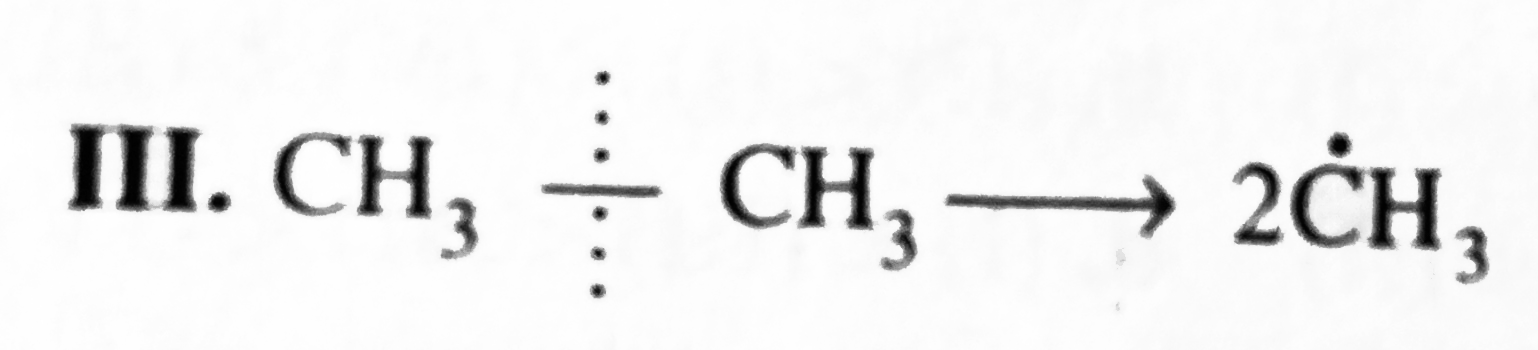 `E_(act) = + 368.0 kJ`
`E_(act) = + 368.0 kJ` 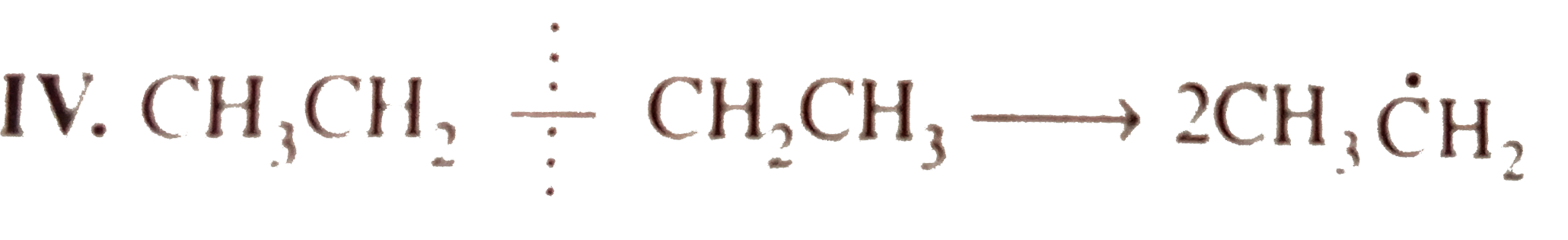 `E_(act) = + 343.0 kJ`
`E_(act) = + 343.0 kJ` 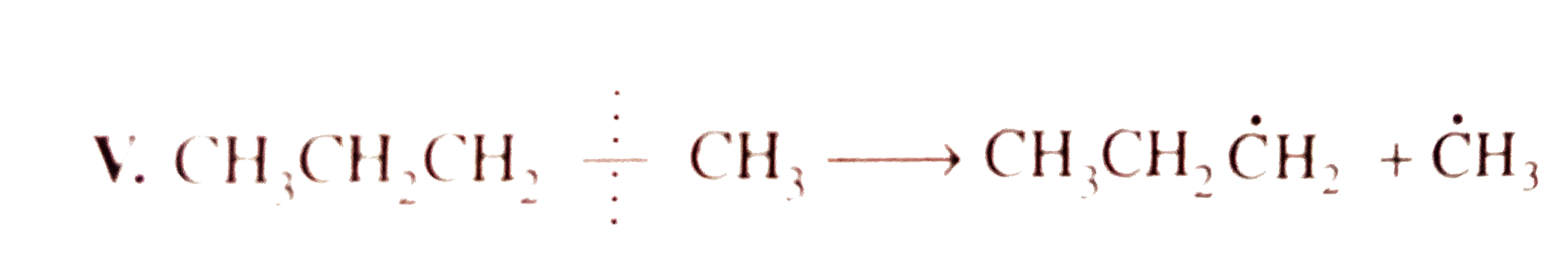 `E_(act) = 355.6 kJ`
`E_(act) = 355.6 kJ`