Similar Questions
Explore conceptually related problems
Recommended Questions
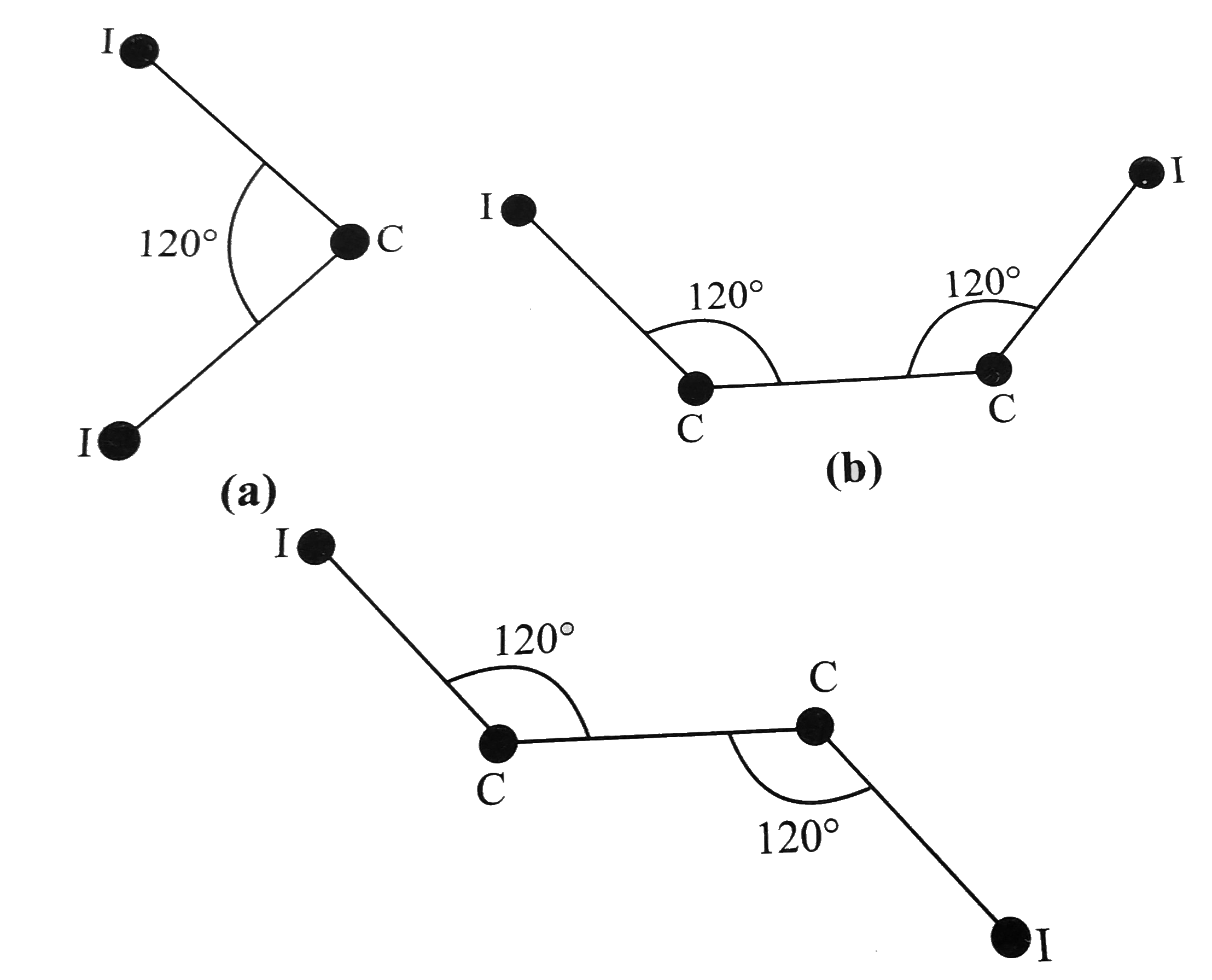
- Calculate the I-I distance in each of the isomeric compounds C(2)H(2)I...
Text Solution
|
- (a) The EN of cesium (Cs) is 0.7 and that of chlorine (Cl) is 3.5 . Pr...
Text Solution
|
- Calculate the I-I distance in each of the isomeric compounds C(2)H(2)I...
Text Solution
|
- Calculate the I-I distance in each of the three isomeric diiodobenzene...
Text Solution
|
- The interatomic distance in H(2) and CI(2) molecules are 74 an d198 pm...
Text Solution
|
- in isolated condition C-C bond length of C(2)H(4) is x than the bond l...
Text Solution
|
- In diamond, the C - C bond lengths are pm.
Text Solution
|
- Bond length of A-A bond is 124 pm and bond length of B-B bond is 174 p...
Text Solution
|
- The C-C bond length in propene is little shorter 149 pm than the C-C b...
Text Solution
|

 .
.