Text Solution
Verified by Experts
Topper's Solved these Questions
IS MATTER AROUND US PURE
DINESH PUBLICATION|Exercise HOTS|6 VideosIS MATTER AROUND US PURE
DINESH PUBLICATION|Exercise MCQ|9 VideosIS MATTER AROUND US PURE
DINESH PUBLICATION|Exercise Short Answer Questions|58 VideosATOMS AND MOLECULES
DINESH PUBLICATION|Exercise LONG ANSWER QUESTIONS|24 VideosMATTER IN OUR SURROUNDINGS
DINESH PUBLICATION|Exercise (LAQs) Long Answer Questions|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-IS MATTER AROUND US PURE-Long Answer Questions
- Define distillation. What type of mixtures can be separated by distill...
Text Solution
|
- You are given a mixture of sand, water and mustard oil. How will you s...
Text Solution
|
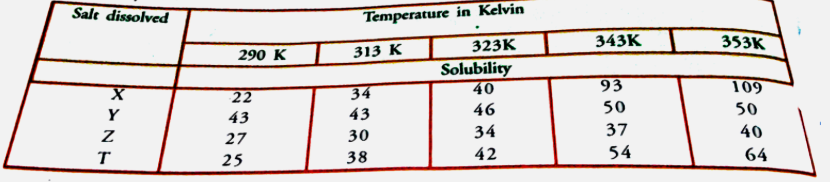
- Sudha tested the solubility of four salts X, Y, Z and T as different t...
Text Solution
|
- (a) Enumerate any two differences between simple distillation and frac...
Text Solution
|
- Why is crystallisation better than evaporation for the separation of m...
Text Solution
|
- Which separation techniques you will apply for the separation of the f...
Text Solution
|
- (a) 'Water is considered as a compound of hydrogen and oxygen and not ...
Text Solution
|
- How will you separate a mixture containing kerosene and petrol (differ...
Text Solution
|
- Explain the terms dilute solution, concentrated solution and saturated...
Text Solution
|
- Observe the apparatus shown and answer the following questions. (a...
Text Solution
|
- What is chromatography ? State its two applications.
Text Solution
|
- (a) Compare metals and non-metals based on their physical properties (...
Text Solution
|
- List four physical properties of metals. Name two metals. Name a metal...
Text Solution
|
- How will you purify an impure sample of copper sulphate containing som...
Text Solution
|
- Give the main points of distinction in true solution, colloidal soluti...
Text Solution
|
- What is Tyndall effect ? How does it help in noticing the particles pr...
Text Solution
|
- A mixture of acetone and methanol can be separated by
Text Solution
|
- What are physical and chemical changes ? How will you distinguish betw...
Text Solution
|
- A mixture whether homogeneous or heterogeneous cannot be a pure substa...
Text Solution
|
- Calculate the mass of sulphuric acid required to prepare its 20% (mass...
Text Solution
|