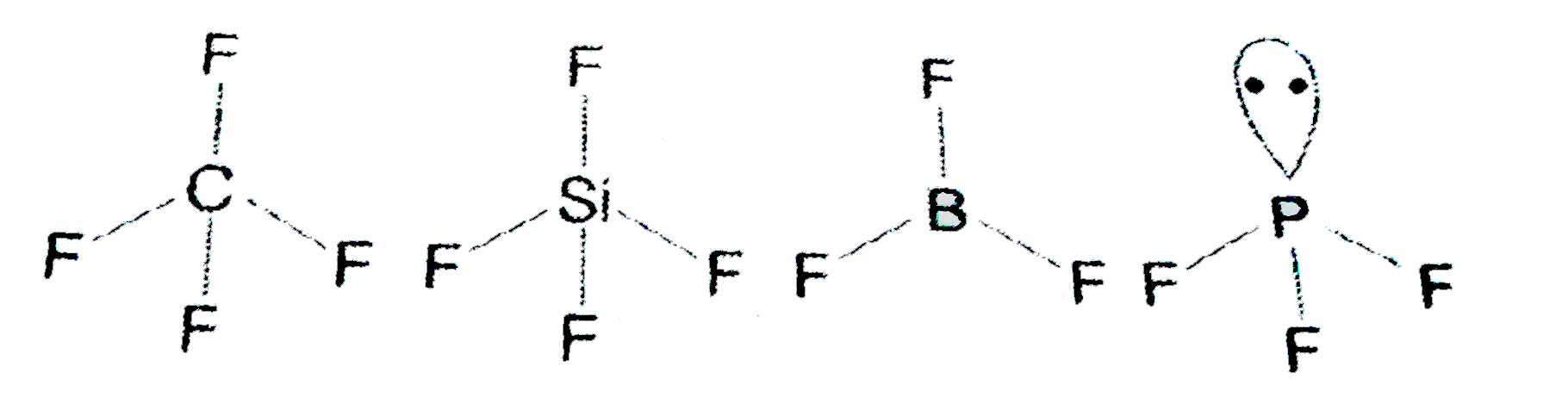
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-IONIC EQUILIBRIUM -All Questions
- The percentage of pyridine (C(5)H(5)N) that forms pyridinium ion (C(5)...
Text Solution
|
- The solubility product of AgCl is 1.8xx10^(-10) at 18^(@)C. The solubi...
Text Solution
|
- Which of the of the following fluoro -compouds is most likely to beahv...
Text Solution
|
- The K(sp) of Ag(2)CrO(4),AgCl,AgBr and AgI are respectively, 1.1xx10^(...
Text Solution
|
- What is the pH of the resulting solution when equal volumes of 0.1 M N...
Text Solution
|
- Which one of the following pairs of solution is not an acidic buffer?
Text Solution
|
- Which of the following salts will give highest pH in water?
Text Solution
|
- Which is the strongest acid in the following ?
Text Solution
|
- Which of these is least likely to act as Lewis base?
Text Solution
|
- Which of the following is electron deficient ?
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- Equimolar solutions of the following substances were prepared separate...
Text Solution
|
- Buffer solutions have constant acidity and alkalinity because
Text Solution
|
- A buffer solution is prepared in which the concentration of NH(3) is 0...
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- What is [H^(+)] in mol//L of a solution that is 0.20 M in CH(3)COONa a...
Text Solution
|
- In a buffer solution containing equal concentration of B^(-) and HB, t...
Text Solution
|
- What is the [OH^(-)] in the final solution prepared by mixing 20.0 mL ...
Text Solution
|
- The ionization constant of ammonium hydroxide is 1.77xx10^(-5) at 298 ...
Text Solution
|
- Which of the following molecules acts as a Lewis acid?
Text Solution
|