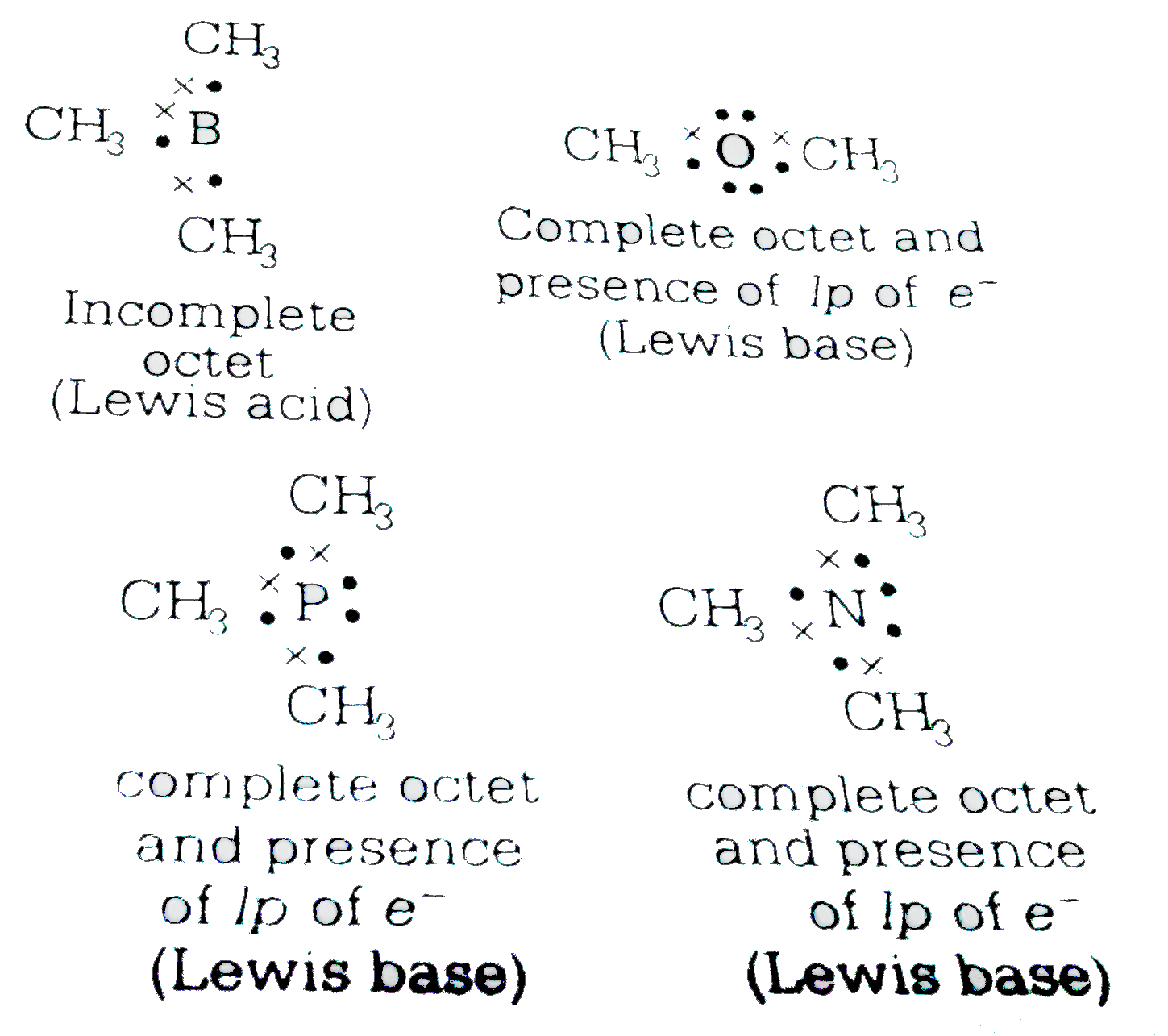A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-IONIC EQUILIBRIUM -All Questions
- What is the [OH^(-)] in the final solution prepared by mixing 20.0 mL ...
Text Solution
|
- The ionization constant of ammonium hydroxide is 1.77xx10^(-5) at 298 ...
Text Solution
|
- Which of the following molecules acts as a Lewis acid?
Text Solution
|
- The values of K(p(1)) and K(p(2)) for the reactions X hArr Y+Z ….(i...
Text Solution
|
- The dissociation equilibrium of a gas AB, can be represented as The d...
Text Solution
|
- Equal volumes of three acid solutions of pH 3, 4 and 5 are mixed in a ...
Text Solution
|
- A weak acid, HA, has a K(a) of 1.00xx10^(-5). If 0.100 mol of the acid...
Text Solution
|
- Calculate the pOH of solution at 25^(@)C that contains 1xx10^(-10) M o...
Text Solution
|
- The hydrogen ion concentration of a 10^(-8) M HCl aqueous soultion at ...
Text Solution
|
- Which of the following constitutes a buffer ?
Text Solution
|
- H(2)S gas when passed through a solution of cations containing HCl pre...
Text Solution
|
- What is the correct relationship between the pH of isomolar solutions ...
Text Solution
|
- At 25^(@)C, the dissociation constant of a base. BOH is 1.0xx10^(-12)...
Text Solution
|
- The rapid change of pH near the stoichiometric point of an acid-base t...
Text Solution
|
- The solubility product of a sparingly soluble salt AX(2) is 3.2xx10^(-...
Text Solution
|
- The solubility product of AgI at 25^(@)C is 1.0xx10^(-16) mol^(2) L^(-...
Text Solution
|
- Solution of 0.1 N NH(4)OH and 0.1 N NH(4)Cl has pH 9.25, then find out...
Text Solution
|
- Which has the highest pH?
Text Solution
|
- Solubility of MX(2) type electrolytes is 0.5xx10^(-4) mol//L, then fin...
Text Solution
|
- Solubility if M(2)S type salt is 3.5xx10^(-6), then find out its solub...
Text Solution
|