Similar Questions
Explore conceptually related problems
Recommended Questions
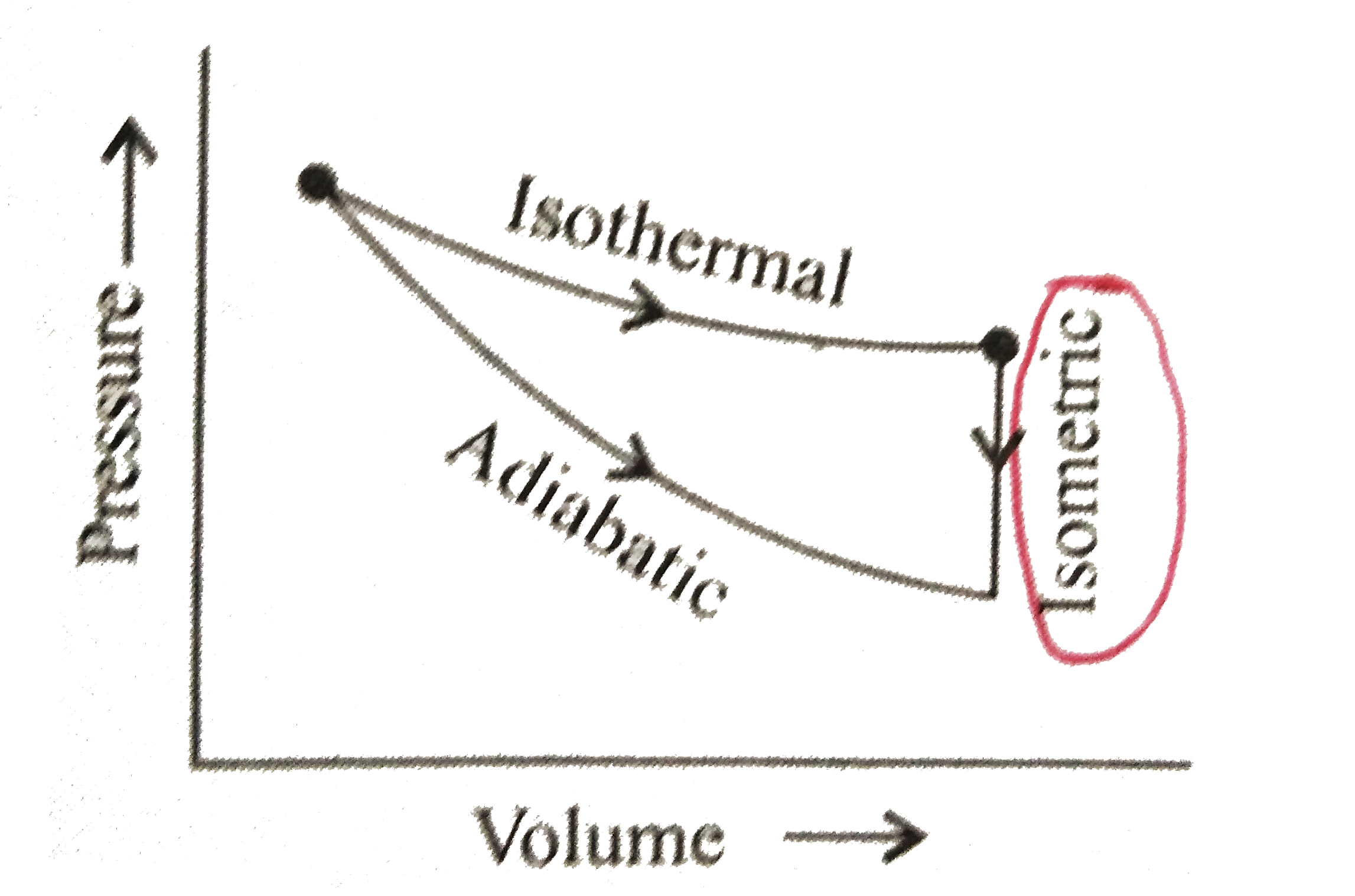
- An ideal diatomic gas is caused to pass throguh a cycle shown on the P...
Text Solution
|
- An ideal diatomic gas is caused to pass throguh a cycle shown on the P...
Text Solution
|
- For an ideal gas, an illustratio of three different paths A(B+C) and (...
Text Solution
|
- For an ideal gas, an illustratio of three different paths A(B+C) and (...
Text Solution
|
- An ideal gasa undergoes through folowing cyclic procees. 1-2 : Reversi...
Text Solution
|
- For an ideal monoatomic gas, an illustration of three different paths ...
Text Solution
|
- An ideal gas passes from one equllibrium state (P(1) ,V(1), T(1), N)...
Text Solution
|
- यदि रुद्धोष्म परिवर्तन में गैस की अवस्था P(1),V(1),T(1) सेP(2),V(2),T(...
Text Solution
|
- An ideal gas expands from state (P(1), V(1)) to state (P(2), V(2)) , w...
Text Solution
|