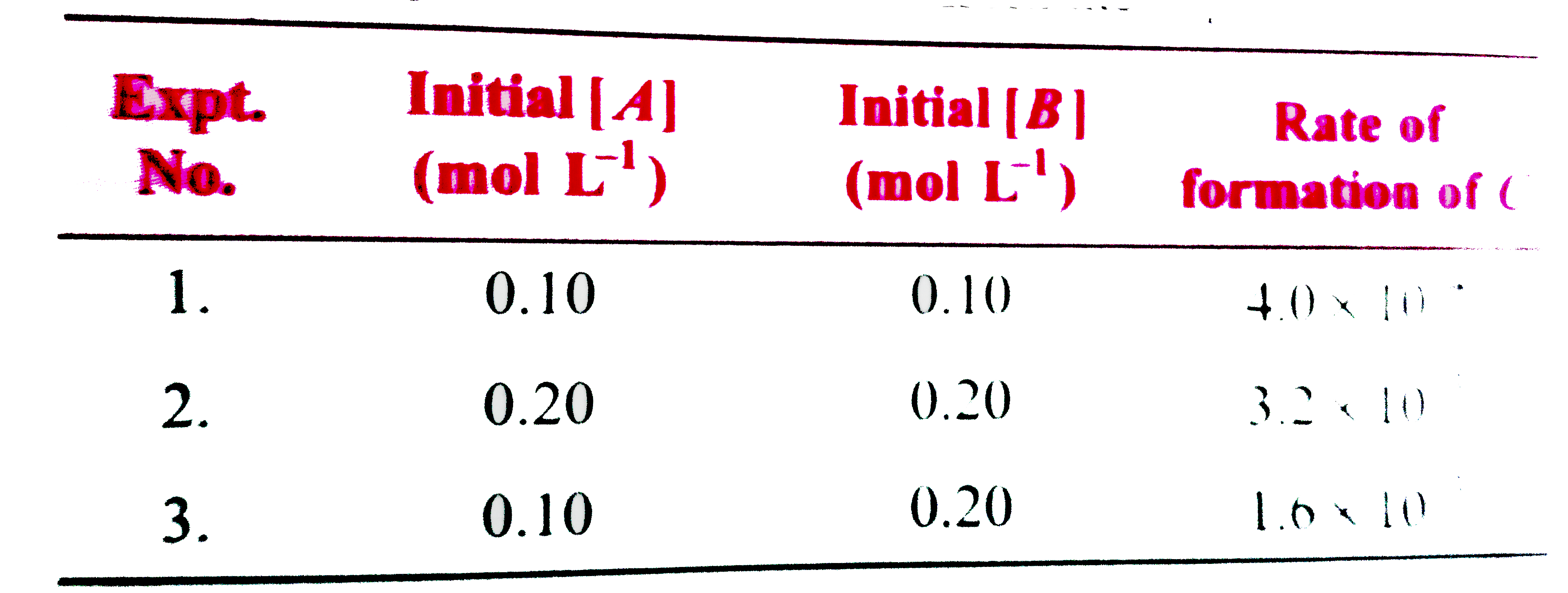Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the rat law expressio for the reaction , A+BrarrC The foll...
Text Solution
|
- The reaction : 2N(2)O(5)(g) to 2NO(2)(g) + (O(2))(g) was studied and t...
Text Solution
|
- For the reaction 2NOrarr2NOCl at 300 K, following data are obtained: W...
Text Solution
|
- What is the rat law expressio for the reaction , A+BrarrC The followin...
Text Solution
|
- The reaction , 2N(2)O(5)rarr4NO(2)+O(2) was studdied and the foll...
Text Solution
|
- The following initial rate data were obtianed for the reaction 2NO...
Text Solution
|
- एक शून्य कोटि की अभिक्रिया A+BrarrC के लिए वेग नियम लिखिए
Text Solution
|
- एक अभिक्रिया की क्रियाविधि निम्नलिखित है, 2A+BrarrD+E A+BrarrC+D ...
Text Solution
|
- नॉन-स्टॉकियोमीट्री अभिक्रिया 2A+BrarrC+D के लिए तीन पृथक प्रयोगो में ...
Text Solution
|