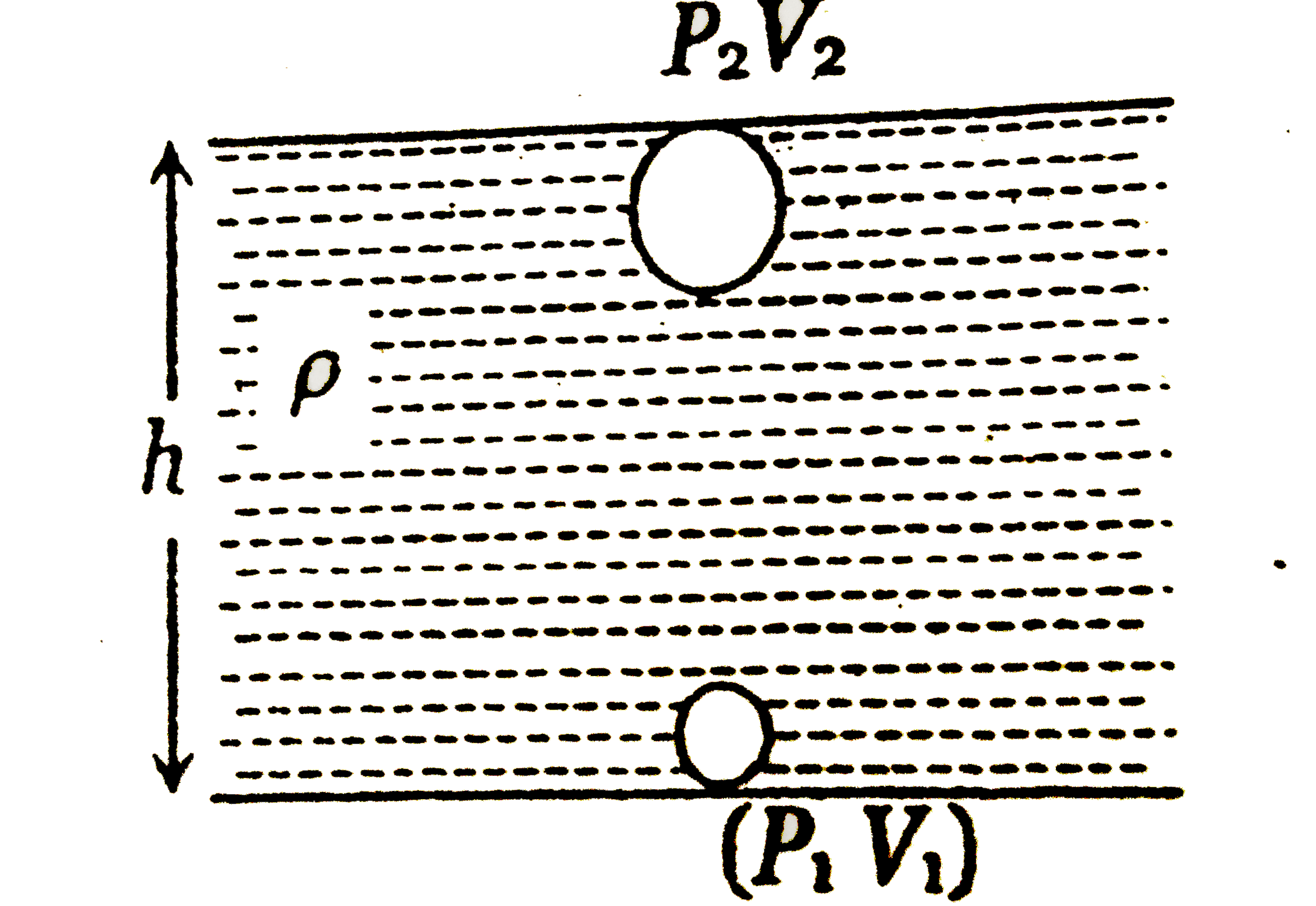
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-FLUID MECHANICS-PYQS AIEEE
- An inverted bell lying at the bottom of a lake 47.6 m deep has 50 cm^3...
Text Solution
|
- A cylinder of height 20m is completely filled with water. The velocity...
Text Solution
|
- Spherical balls of radius 'R' are falling in a viscous fluid of viscos...
Text Solution
|
- If two soap bubbles of different radii are connected by a tube
Text Solution
|
- A 20cm long capillary tube is dipped in water. The water rises up to 8...
Text Solution
|
- If the terminal speed of a sphere of gold (density =19.5kg//m^3) is 0....
Text Solution
|
- A spherical solild of volume V is made of a material of density rho(1)...
Text Solution
|
- A jar filled with two non-mixing liquid 1 and 2 having densities rho(1...
Text Solution
|
- A capillary tube (A) is dipped in water. Another identical tube (B) is...
Text Solution
|
- A ball is made of a material of density rho where rho(oil)ltrholtrho(w...
Text Solution
|
- A thin liquid film formed between a U-shaped wire and a light slider s...
Text Solution
|