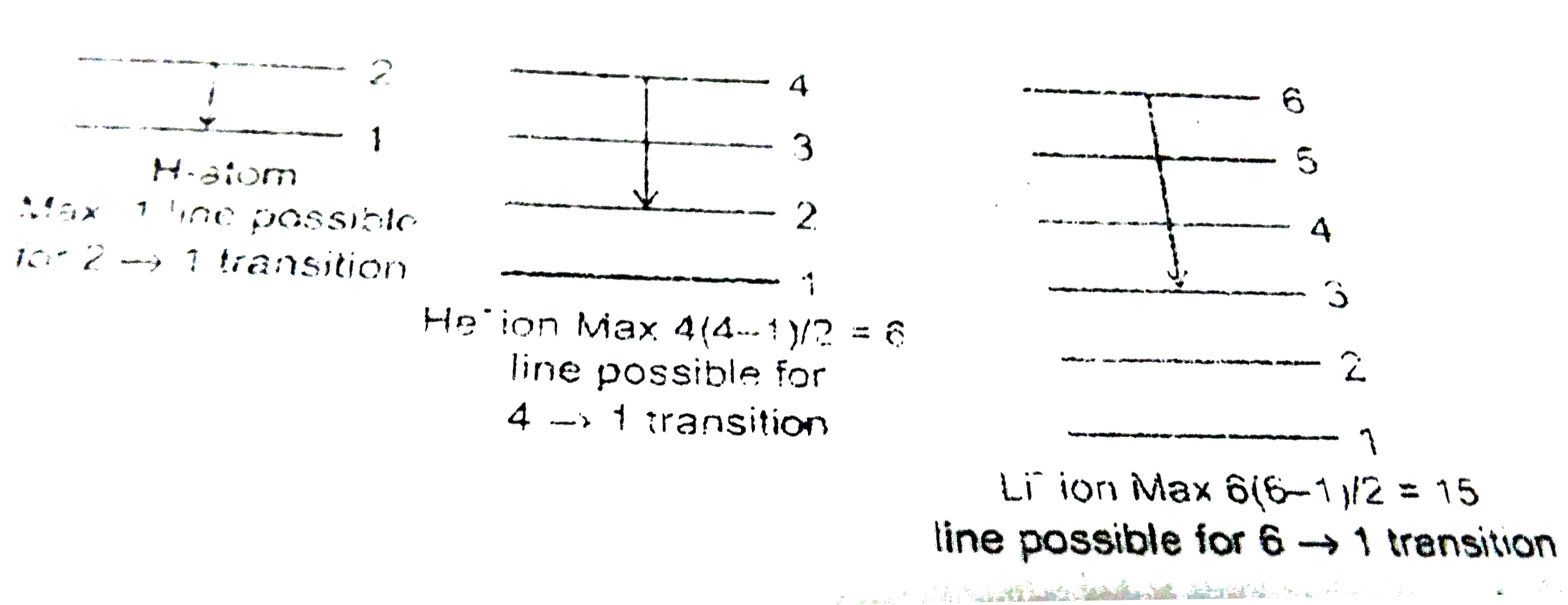Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
RESONANCE|Exercise PART -II|32 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise Exercise-2|25 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise Board Level Exercise|39 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NUCLEAR CHEMISTRY-Exercise-1
- At what atomic number would a transition from n=2"to"n=1 energy level ...
Text Solution
|
- Calculate the energy emitted when electron of 1.0 gm atom of Hydroge...
Text Solution
|
- In a container a mixture is prepared by mixing of three samples of hyd...
Text Solution
|
- An electron in H-atom in its ground state absorbs 1.5 times as much e...
Text Solution
|
- Deduce the condition when the De-Broglie wavelength associated with an...
Text Solution
|
- An electron, practically at rest, is initially accelerated through a p...
Text Solution
|
- If an electron having kinetic energy 2 eV is accelerated through the p...
Text Solution
|
- The uncertainty in position and velocity of the particle are 0.1 nm an...
Text Solution
|
- An electron moving near an atomic nucleus has a speed of 6xx10^(6) +- ...
Text Solution
|
- An electrons in a hydrogen atom finds itself in the fourth energy leve...
Text Solution
|
- The wave function of 3s electron is given by Psi(3s)=1/(81sqrt(3)pro...
Text Solution
|
- How many unpaired electrons are there in Ni^(2+)?
Text Solution
|
- Write the electronic configuration of the element having atomic number...
Text Solution
|
- Given below are sets of quantum numbers for given orbitals. Name these...
Text Solution
|
- Point out the anugular momentum of an electron in, (a) 4s orbital (b...
Text Solution
|
- Which of the following sets of quantum numbers are impossible for elec...
Text Solution
|
- Find the total spin and spin magnetic moment of following ion. (i) F...
Text Solution
|
- Calculate the loss in mass during the change: .(3)Li^(7) + .(1)He^(1...
Text Solution
|
- When ^(24)Mg is bombared with neutron then a proton is ejected. Comple...
Text Solution
|
- Write equations for the following transformation: (a) .(7)^(17)N (n,...
Text Solution
|