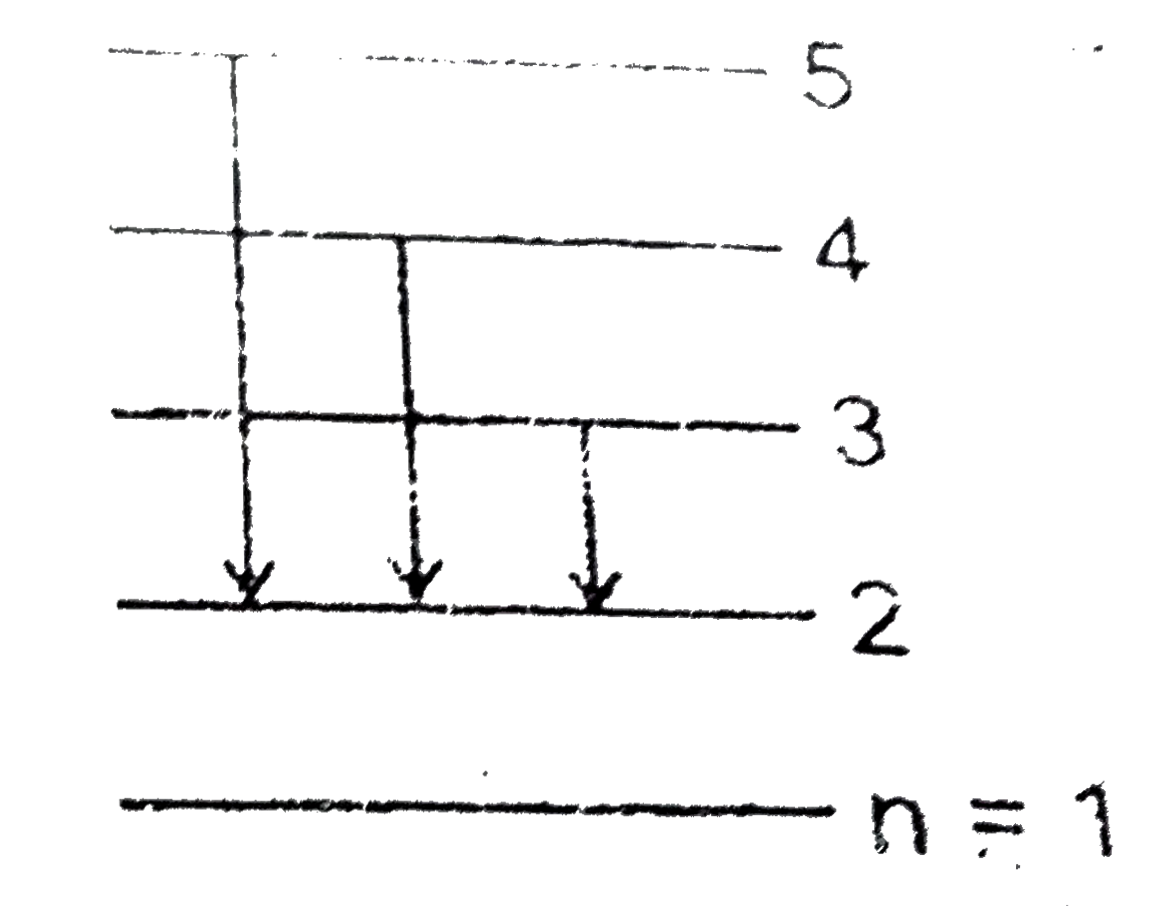
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a H-like sample, electrons make transition from 4^(th) excited stat...
Text Solution
|
- As an electron makes a transition from an excited state to the ground ...
Text Solution
|
- As an electron makes a transition from an excited state to the ground ...
Text Solution
|
- In a H- like sample electron makes transition from 4th excited state o...
Text Solution
|
- In a sample of three H-atoms , all in the 5th excited state, electrons...
Text Solution
|
- In a sample of H-atoms electrons are de-exicited from 4^(th) excited s...
Text Solution
|
- The photon radiated from hydrogen corresponding to 2^(nd) of Lyman ser...
Text Solution
|
- If electron make transition from 7^(th) excited state to 2^(nd) state ...
Text Solution
|
- In a H-like sample, electrons make transition from 4^(th) excited stat...
Text Solution
|