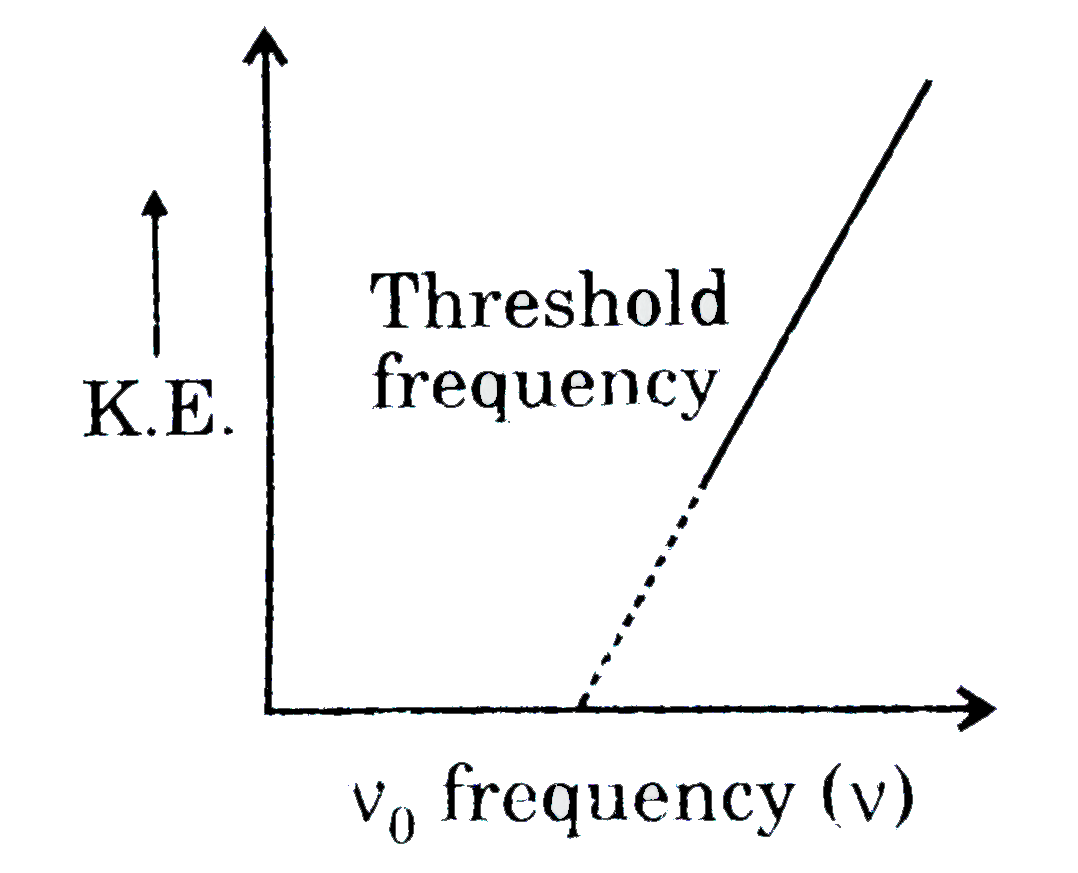
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
RESONANCE|Exercise Exercise-3|15 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise PART-II|26 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise PART -III :|8 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 Videos
RESONANCE-NUCLEAR CHEMISTRY-PART-IV : COMPREHENSION
- In the photoelectric effect the eletrons are emiited intantaneously fr...
Text Solution
|
- In the photoelectric effect the eletrons are emiited intantaneously fr...
Text Solution
|
- In the photoelectric effect the eletrons are emiited intantaneously fr...
Text Solution
|
- the only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- the only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- the only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- de Broglie proposed dual nature for electron by putting his famous equ...
Text Solution
|
- de Broglie proposed dual nature for electron by putting his famous equ...
Text Solution
|
- de Broglie proposed dual nature for electron by putting his famous equ...
Text Solution
|
- After the faliure of Bohr atomic theory but its ability to explain to ...
Text Solution
|
- After the faliure of Bohr atomic theory but its ability to explain to ...
Text Solution
|
- Quantum number area assigned to get complete information of electron...
Text Solution
|
- Quantum number area assigned to get complete inforamtion of electron...
Text Solution
|
- Quantum number area assigned to get complete inforamtion of electron...
Text Solution
|