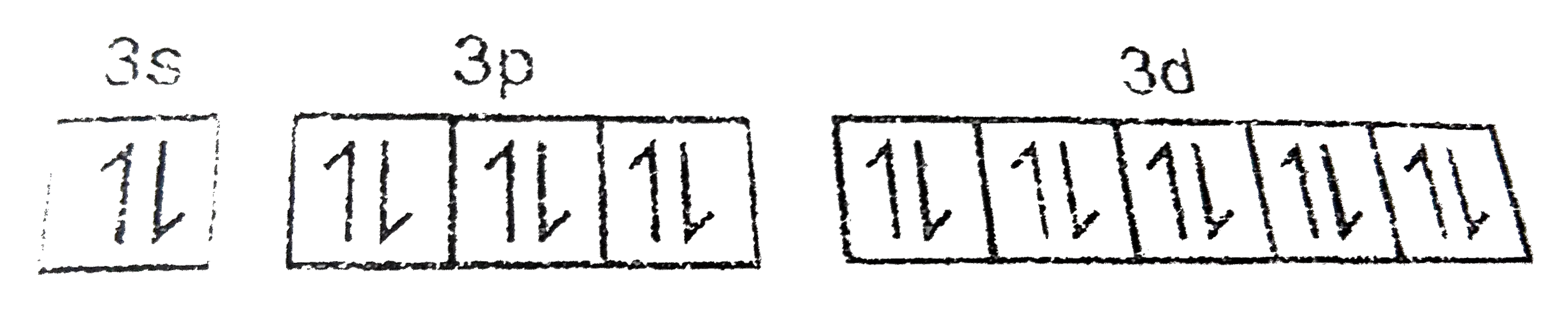Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
RESONANCE|Exercise PART-II|26 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise ADVANCED LEVEL PROBLEMS|30 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise PART-IV : COMPREHENSION|14 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NUCLEAR CHEMISTRY-Exercise-3
- Calculated the energy required to excite one litre of hydrogen gas at ...
Text Solution
|
- The orbit having Bohr radius equal to 1st Bohr orbit of H-atom is :
Text Solution
|
- (a) The wave function of an electron in 2s orbital in hydrogen atom is...
Text Solution
|
- a.Calculate the velocity of an electron in the first Bohr's orbit of ...
Text Solution
|
- The hydrogen -like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|
- The hydrogen -like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|
- The hydrogen -like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|
- The work function (phi) of some metals is listed below. The number of...
Text Solution
|
- The maximum number of electrons that can have principal quantum number...
Text Solution
|
- Bombardment of aluminium by alpha-"particle" leads to its artificial d...
Text Solution
|
- The kinetic energy of an electron in the second Bohr orbit of a hydrog...
Text Solution
|
- The periodic table consists of 18 groups. An isotope of copper, on bom...
Text Solution
|
- In the nuclear transmutation : .(4)^(9) Be + X rarr (4) ^(8) Be +Y ...
Text Solution
|
- In an atom, the total number of electrons having quantum numbers n =...
Text Solution
|
- Not considering the electronic spin, the degeneracy of the second exci...
Text Solution
|