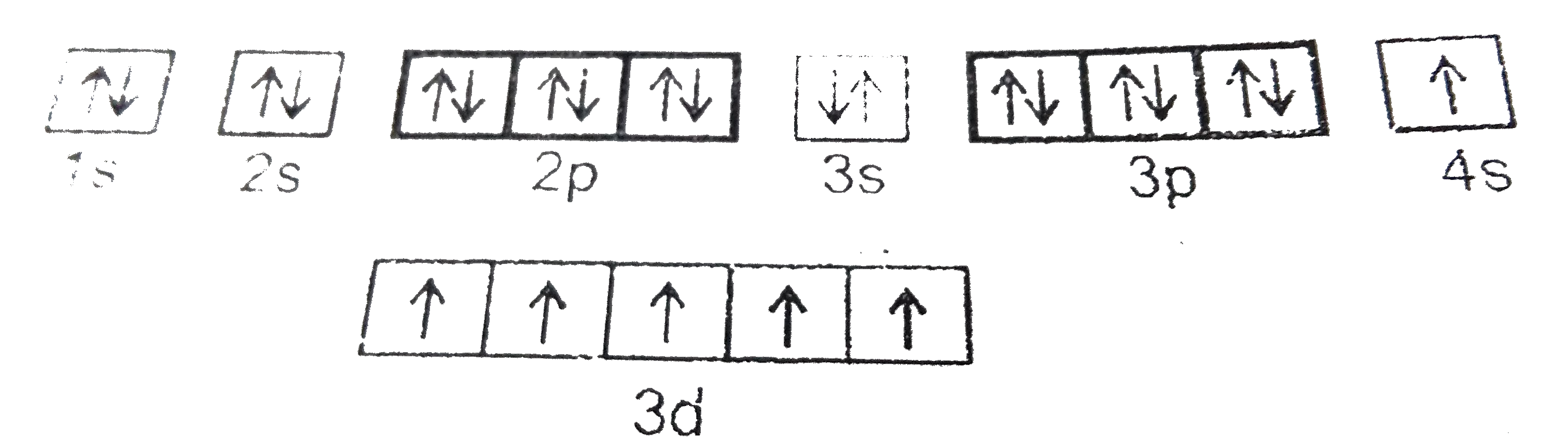
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
RESONANCE|Exercise PART-II SECTION-1|21 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise PART-III|5 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise PART-II|26 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NUCLEAR CHEMISTRY-ADVANCED LEVEL PROBLEMS
- The increasing order (lowest first) for the values of e//m (charge//ma...
Text Solution
|
- An electron in an atom jumps in such a way that its kinetic energy cha...
Text Solution
|
- What atomic number of an element "X" would have to become so that the ...
Text Solution
|
- Select the incorrect graph for velocity of e^(-) in an orbit vs. Z, 1/...
Text Solution
|
- Which of the following is discreted in Bohr's theory?
Text Solution
|
- The mass of a proton is 1836 times more than the mass of an electron. ...
Text Solution
|
- In any subshell, the maimum number of electrons having same value of s...
Text Solution
|
- Which quantum number defines the orientation of orbital in the space a...
Text Solution
|
- For similar orbitals having different values of n:
Text Solution
|
- Maximum number of nodes are present in :
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- Which of the following has the maximum number of unpaired electrons?
Text Solution
|
- The angular velocity of an electron occupying the second Bohr orbit of...
Text Solution
|
- An excited state of H atom emits a photon of wavelength lamda and retu...
Text Solution
|
- Light of wavelength lambda strikes a metal surface with intensity X an...
Text Solution
|
- Neutron scattering experiments have shown that the radius of the nucle...
Text Solution
|
- The nucleus of an atom is located at x=yx=z=0. If the probability of f...
Text Solution
|
- The energy of a I,II and III energy levels of a certain atom are E,(4E...
Text Solution
|
- A compound of vanadium has a magnetic moment of 1.73 BM. What will be ...
Text Solution
|
- Calculate the minimum and maximum number of electrons which may have m...
Text Solution
|