A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
RESONANCE|Exercise PART-III|5 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise ADVANCED LEVEL PROBLEMS|30 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NUCLEAR CHEMISTRY-PART-II SECTION-1
- For a 3s-orbital Phi(3s)=(1)/(asqrt(3))((1)/(a(0)))^(3//2)(6-6sigma+...
Text Solution
|
- A glow-worm of mass 5.0 g emits red light (650 nm) with a power of 0.1...
Text Solution
|
- Calculate the energy required to excited one litre of hydrogen gas at ...
Text Solution
|
- O(2) undergoes photochemical dissociation into one normal oxygen a...
Text Solution
|
- If the subsidiary quantum number of a subenergy level is 4, the maximu...
Text Solution
|
- 1 mol of He^(+) ion is excited. Spectral analysis showed existence of ...
Text Solution
|
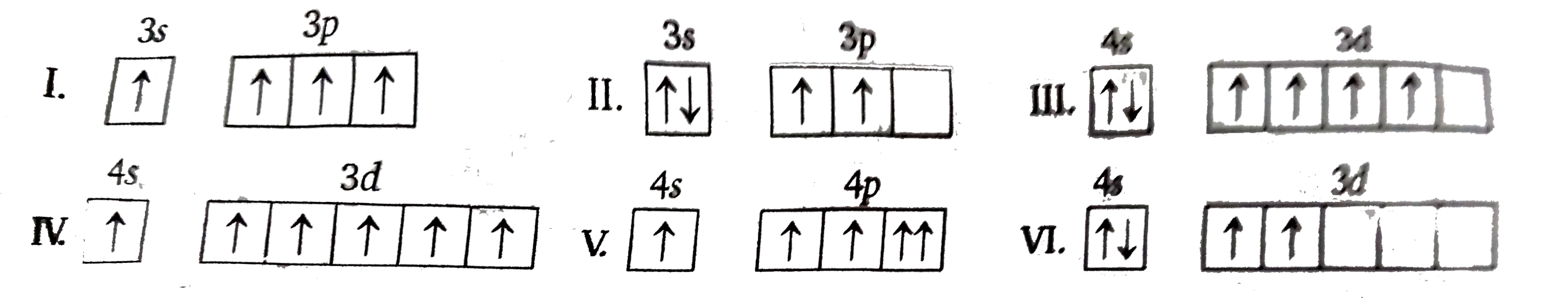
- Consider the following six electronic configurations (remaining inner ...
Text Solution
|
- Choose the correct statement (s) :
Text Solution
|
- The radial distribution functions [P(r)] is used to determine the most...
Text Solution
|
- For radial probability curves. Which of the following is/are correct ?
Text Solution
|
- d(xy) orbital has lobes between x- and y- axes. The wave function of t...
Text Solution
|
- The dissociation energy of H(2) is 430.53 kJ mol^(-1), If H(2) is of d...
Text Solution
|
- The IE(1) of H is 13.6 eV. It is exposed to electromagnetic waves of 1...
Text Solution
|
- A hydrogen like atom (atomic number z) is in a higher excited state of...
Text Solution
|
- A moving particle is associated with wavelength 5xx10^(-8) m. If its m...
Text Solution
|
- According to Bohr's theory, the electronic energy of an electron in t...
Text Solution
|
- .(4)Be^(7) captures a K electron into its nucleus .What is the mass ...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|