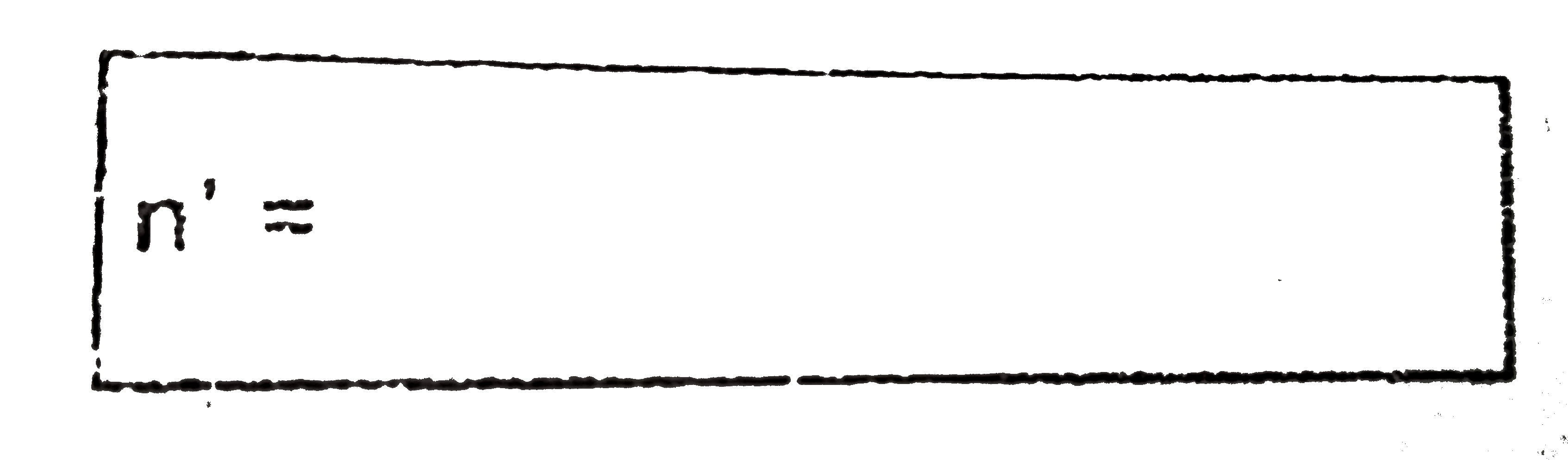
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydrogen atom: The electronic ground state of hydrogen atom contains...
Text Solution
|
- What is the energy in eV requried to excite the electron from n=1 to n...
Text Solution
|
- If R(H) is the Rydberg constant, then the energy of an electron in the...
Text Solution
|
- Hydrogen atom: The electronic ground state of hydrogen atom contains o...
Text Solution
|
- Hydrogen atom: The electronic ground state of hydrogen atom contains o...
Text Solution
|
- Hydrogen atom: The electronic ground state of hydrogen atom contains o...
Text Solution
|
- Hydrogen atom: The electronic ground state of hydrogen atom contains...
Text Solution
|
- Hydrogen atom: The electronic ground state of hydrogen atom contains o...
Text Solution
|
- Hydrogen atom: The electronic ground state of hydrogen atom contains...
Text Solution
|