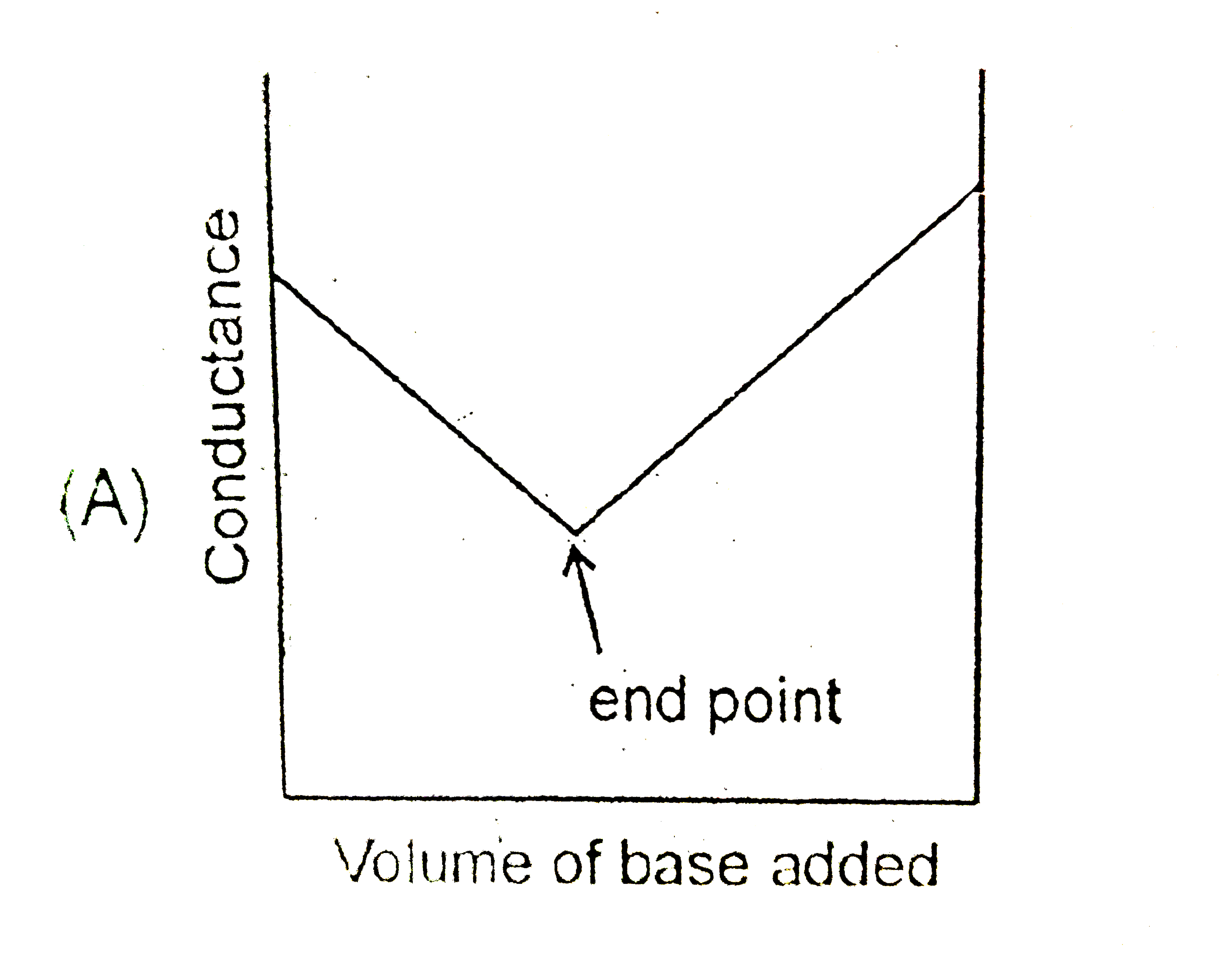
A
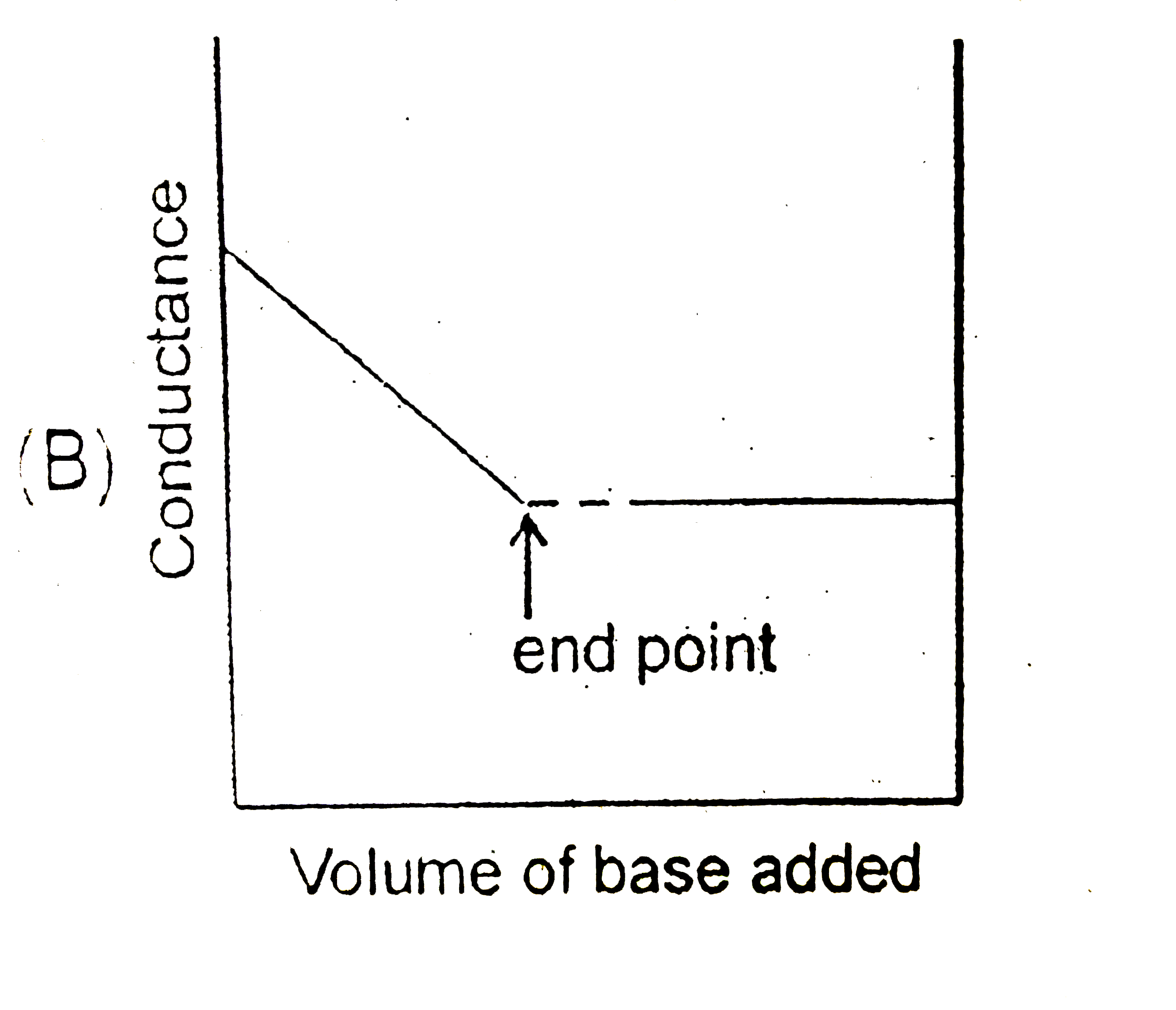
B
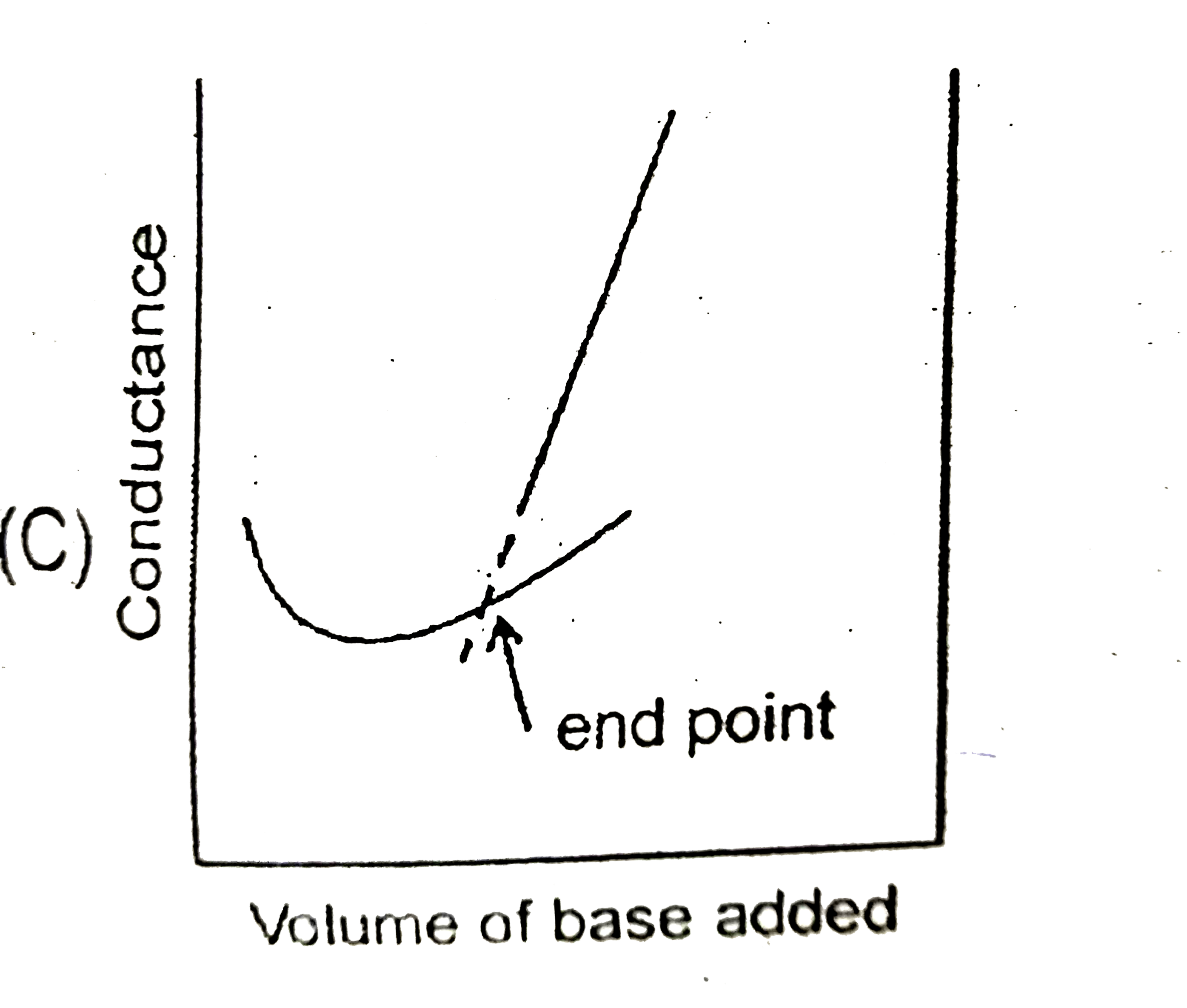
C
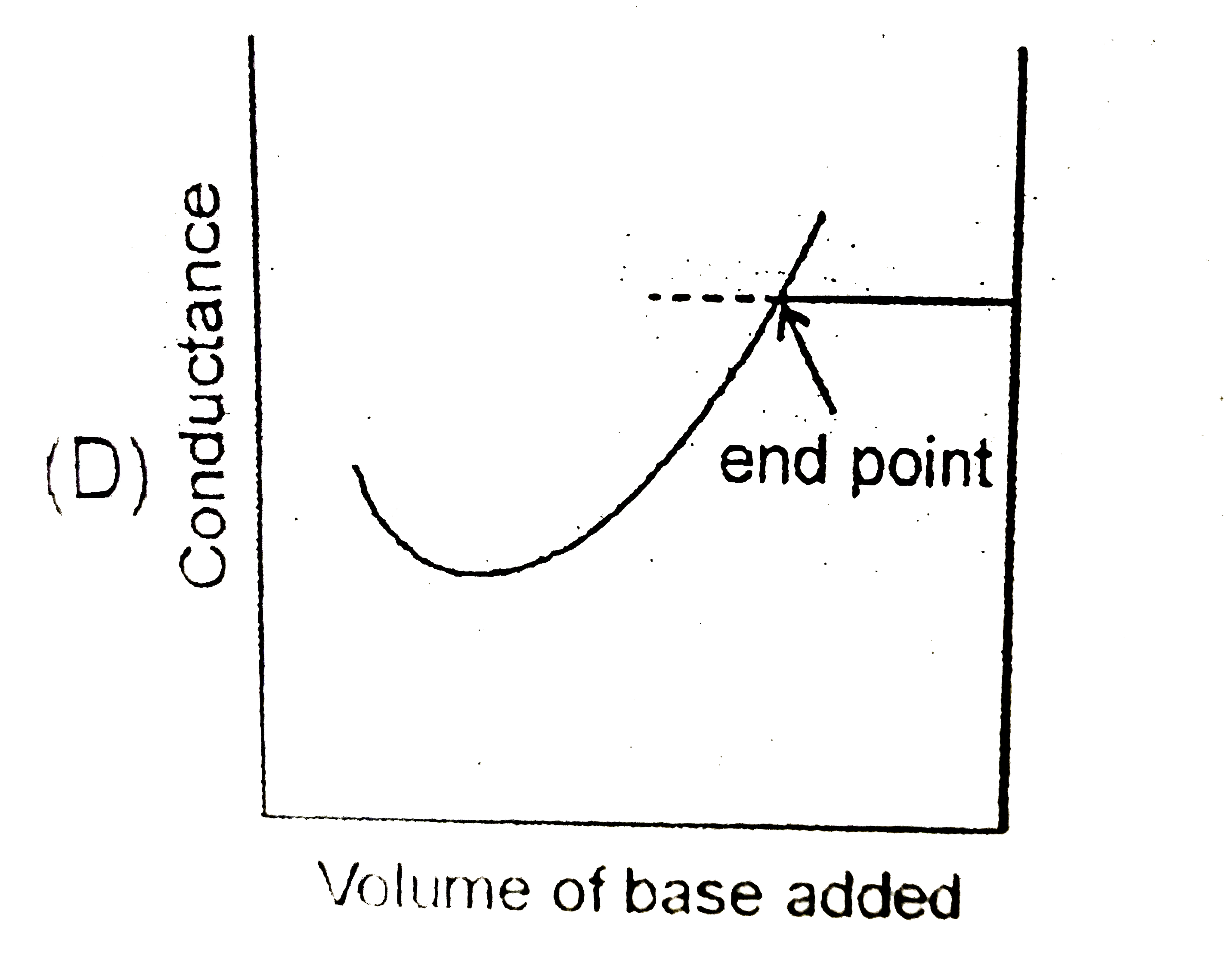
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISRY
RESONANCE|Exercise Comprehension|31 VideosELECTROCHEMISRY
RESONANCE|Exercise Exercise 3|7 VideosELECTROCHEMISRY
RESONANCE|Exercise Subjective Questions|14 VideosELECTRO CHEMISTRY
RESONANCE|Exercise PHYSICAL CHEMITRY (ELECTROCHEMISTRY)|53 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE|Exercise Part -IV|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-ELECTROCHEMISRY-Objective Questions
- Pure water is saturated with pure solid AgCl, a silver electrode is pl...
Text Solution
|
- The two aqueous solutions, A (AgNO3) and B (LiCI), were electrolysed u...
Text Solution
|
- When iron is rusted, it is
Text Solution
|
- Which statement is correct?
Text Solution
|
- Conductance measurements and be used to detect the end point of acid-b...
Text Solution
|
- Using the standard electrode potential values given below, decide whi...
Text Solution
|
- When the electric current is passed through a cell having an electroly...
Text Solution
|
- Which one of the following will increase the voltage of the cell ? (T...
Text Solution
|
- In H2 -O2 fuel cell, 6.72 L of hydrogen at NTP reacts in 15 minutes, ...
Text Solution
|
- Four moles of electrons were transferred from anode to cathode in an e...
Text Solution
|
- the standard reduction potenital of a silver chloride electrode is 0.2...
Text Solution
|
- A cell Cu|Cu^(++)||Ag^(+)|Ag initially contains 2MAg^(+) and 2MCu^(++)...
Text Solution
|
- With t taken in seconds and I taken in amp, the variation of I follows...
Text Solution
|
- When a cleaned strip of zinc metal is placed in a solution of CuSO(4),...
Text Solution
|
- During electrolysis of aqueous CuBr(2) using Pt electrode,
Text Solution
|
- A current of 2.68 A is passed for 1.0 hour through an aqueous solution...
Text Solution
|
- Mark out the correct statement(s).
Text Solution
|
- Mark out the correct statement(s) regarding electrolytic molar conduct...
Text Solution
|
- If same quantity of electricity is passed through three electrolytic c...
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|