A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-ELECTROCHEMISRY-Advanced Level Problems
- If the pressure of hydrogen gas is increased from 1 atm. To 100 atm, k...
Text Solution
|
- The equilibrium Cu^(+2)(aq)+Cu(s) hArr2Cu^(+) established at 20^(@)C...
Text Solution
|
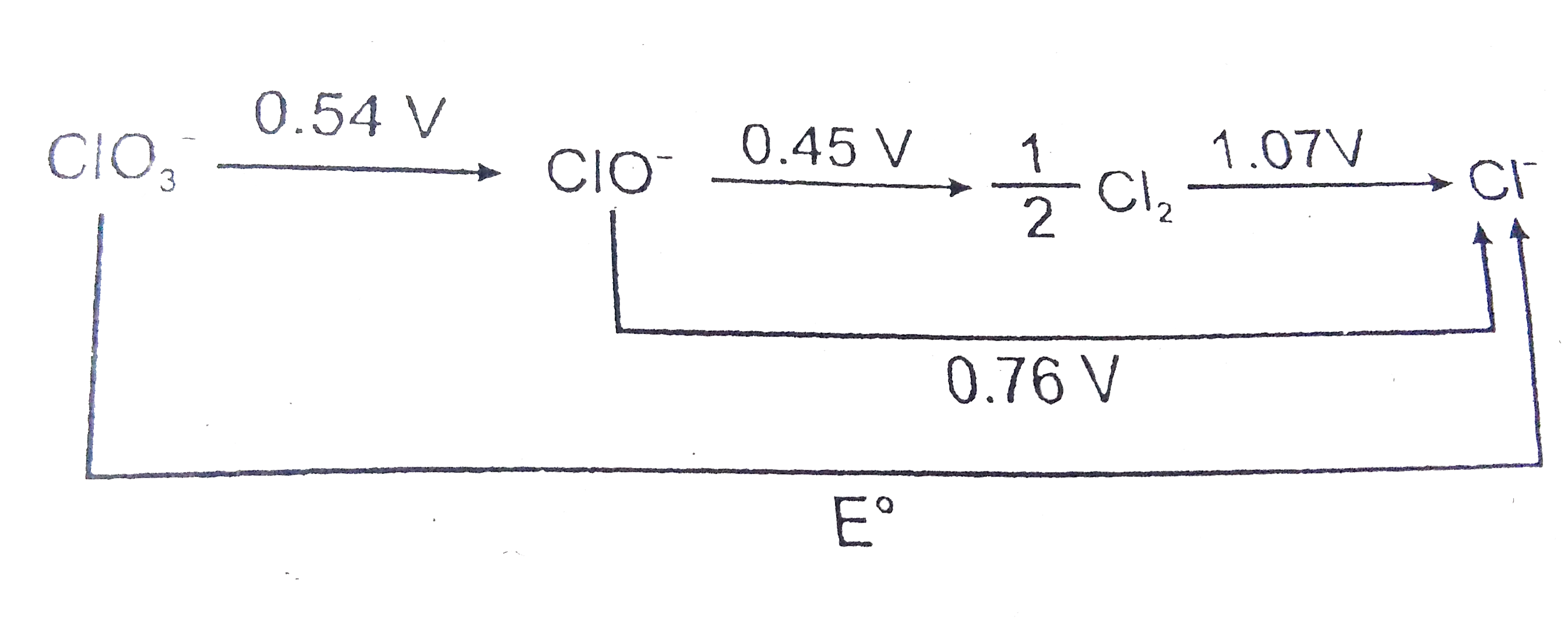
- The E^(@) in the given figure is
Text Solution
|
- What is the cell entropy change ( in J K ^(-1)) of the following cell...
Text Solution
|
- Na- amalgam is prepared by electrolysis of NaCl solution using liquid...
Text Solution
|
- Zn amalgam is prepared by electrolysis of aqueous ZnCl(2) using Hg cat...
Text Solution
|
- A solution containing one mole per litre each of Cu(NO(3))(2),AgNO(3),...
Text Solution
|
- At 298 K the standard free energy of formation of H(2)O(l) is -237.20 ...
Text Solution
|
- Wlhich of the following cell can produce more electrical work?
Text Solution
|
- A hydrogen electrode is immersed in a solution with pH = 0 (HCl). By h...
Text Solution
|
- A current of 1.0 A was passed for 2 hr through a solution of cuprocyan...
Text Solution
|
- A resistance of 50Omega is registered when two electrodes are suspende...
Text Solution
|
- Calcualte the cell EMP in mV for Pt|H(2)(1 atm) | HCl(0.01 M)|| AgCl...
Text Solution
|
- Calculate the value of Lambda(m) ^prop for SrCl(2) in water at 25^(@)...
Text Solution
|
- A current is passed through 2 voltmeters connected in series. The firs...
Text Solution
|
- Which of the following is not true about emf of a cell?
Text Solution
|
- The number of electrons delivered at the cathode during electrolysis b...
Text Solution
|
- By the electrolysis of aqueous solution of CuSO(4), the products obtai...
Text Solution
|
- How many gm of silver will be displaced from a solution of AgNO(3) by ...
Text Solution
|
- The "mole"s of electrons required to deposit 1 gm equivalent aluminium...
Text Solution
|