Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Illustration|18 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise problem for practise|31 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|4 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Integer
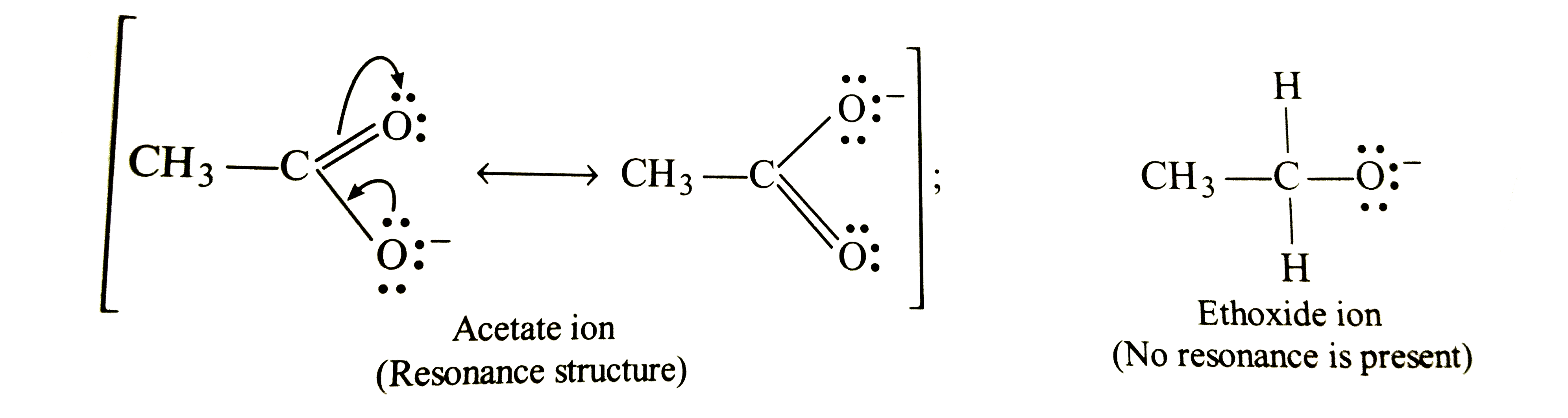
- Explain: (a) Acetic Acid is a stronger acid than ethyl alcohol (b) T...
Text Solution
|
- How many different condensation products would be form by the given re...
Text Solution
|
- How many of the following compounds can show Claisen condensation reac...
Text Solution
|
- How many of following esters show acid catalysed unimolecular and alky...
Text Solution
|
- How many of the following compounds are anhydrides?
Text Solution
|