Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
RESONANCE|Exercise (MSPs)|8 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Board Level Exercise|33 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Advanced Level Problems (Part-3)(Stage-5)|2 VideosCHEMICAL BONDING
RESONANCE|Exercise Inorganic chemistry (Chemistry Bonding)|49 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Match the column|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-CHEMICAL EQUILIBRIUM-Solved examples
- (1) Consider the following cases- The nature of flow of energy ...
Text Solution
|
- For the reaction NOBr (g)iffNO(g)+(1)/(2)Br(2)(g) K(P)=0.15 atm at 9...
Text Solution
|
- The K(P) values for three reactions are 10^(-5), 20 and 300 then what ...
Text Solution
|
- In an experiment starting with 1 mol C(2)H(5)OH, 1 mol CH(3)COOH, and ...
Text Solution
|
- Calculate the degree of dissociation and K(P) for the following reacti...
Text Solution
|
- The vapour density of a mixture containing NO(2) " and "N(2)O(4) is 38...
Text Solution
|
- The equilibrium constant of the reaction at 25^(@)C CuSO(4).5H(2)O(s...
Text Solution
|
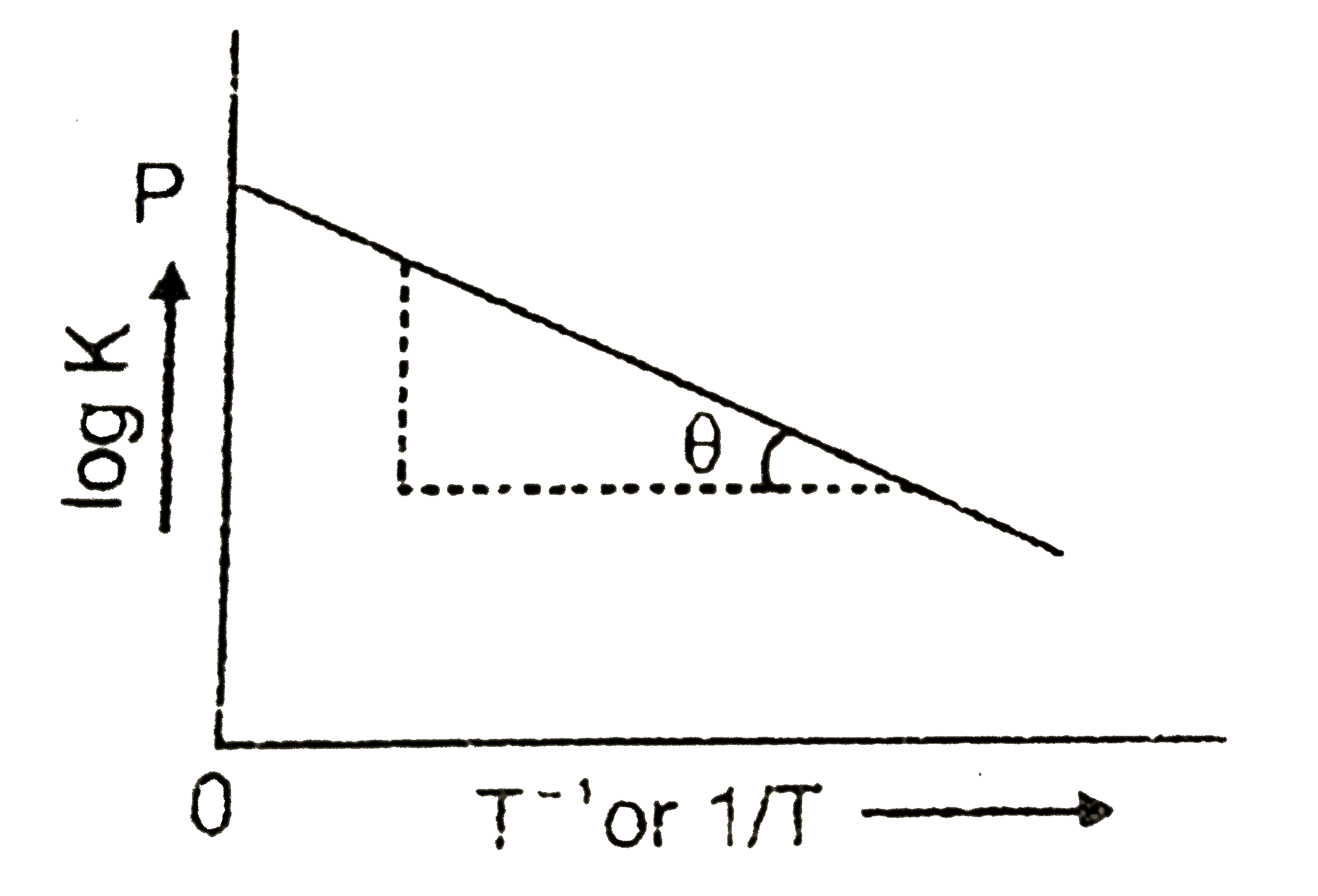
- Variation of equiibrium constant K with temperature T is given by van ...
Text Solution
|
- 102 g of solid NH(4)HS is taken in the 2L evacuated flask at 57^(@). F...
Text Solution
|