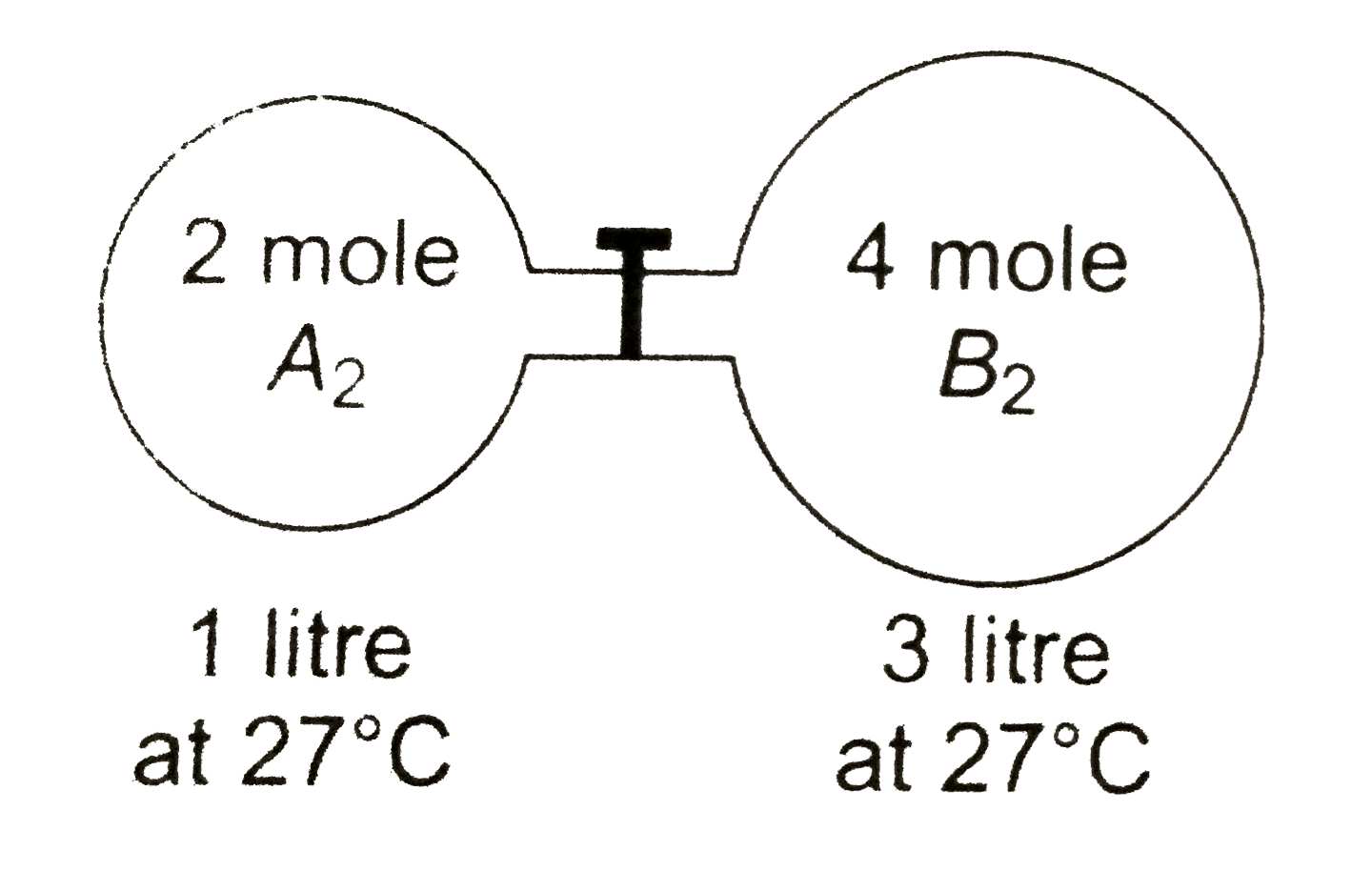Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
RESONANCE|Exercise Exercise-1 (Part-2)|88 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Exercise-2 (Part-1)|28 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Board Level Exercise|33 VideosCHEMICAL BONDING
RESONANCE|Exercise Inorganic chemistry (Chemistry Bonding)|49 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Match the column|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-CHEMICAL EQUILIBRIUM-Exercise-1 (Part-1)
- The equilibrium constant of the reaction A(2)(g)+B(2)(g)hArr2AB(g) at ...
Text Solution
|
- For the reaction 3A(g)+B(g) hArr 2C(g) at a given temperature , Kc=9.0...
Text Solution
|
- The gas A(2) in the left flask allowed to react with gas B(2) present ...
Text Solution
|
- n mole each of H(2)O(g),H(2)(g) "and" O(2)(g) are mixed at a suitable ...
Text Solution
|
- The partial pressures of N(2)O(4) "and" NO(2) "at" 40^(@)C for the fol...
Text Solution
|
- 1 "mole of" N(2) "and" 3 "moles of " H(2) are placed in 1L vessel. Fin...
Text Solution
|
- Calculate the expression for K(C) "and" K(P) if initially a moles of N...
Text Solution
|
- 1 mole of a gas A is taken in a vessel of volume 1L. It dissociates ac...
Text Solution
|
- 0.15 mole of CO taken in a 2.5L flask is maintained at 750K along with...
Text Solution
|
- A mixture of 1.57 mol of N(2), 1.92 mol of H(2) and 8.13 mol of NH(3) ...
Text Solution
|
- At 460^(@)C, K(C)=81 for the reaction, SO(2)(g)+NO(2)(g)hArrNO(g)+SO(3...
Text Solution
|
- Explain the effect of the following on the equilibrium constant. (i)...
Text Solution
|
- The equilibrium constant for the reactions N(2)+O(2)hArr2NO"and "NO+...
Text Solution
|
- Calculate the equilibrium constant for the reaction, H(2(g))+CO(2(g)...
Text Solution
|
- The homogeneous reversible reaction, C(2)H(5)OH+COOHhArrCH(3)COOC(2)H(...
Text Solution
|
- The ester, ethyl acetate is formed by the reaction between ethanol and...
Text Solution
|
- N(2)O(4) is 25% dissociated at 37^(@)C and one atmosphere pressure. Ca...
Text Solution
|
- At temperature T, a compound AB(2)(g) dissociates according to the rea...
Text Solution
|
- Vapour density of the equilibrium mixture of NO(2) "and" N(2)O(4) is f...
Text Solution
|
- When sulphur in the form of S(8) is heated at 900 K, the initial press...
Text Solution
|