A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
RESONANCE|Exercise Exercise-2 (Part-1)|28 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Exercise-2 (Part-2)|23 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Exercise-1 (Part-1)|38 VideosCHEMICAL BONDING
RESONANCE|Exercise Inorganic chemistry (Chemistry Bonding)|49 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Match the column|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-CHEMICAL EQUILIBRIUM-Exercise-1 (Part-2)
- In a reversible reaction Aunderset(K2)overset(K1)hArrB the initial con...
Text Solution
|
- The reaction A + B hArr C + D is studied in a one litre vessel at 250^...
Text Solution
|
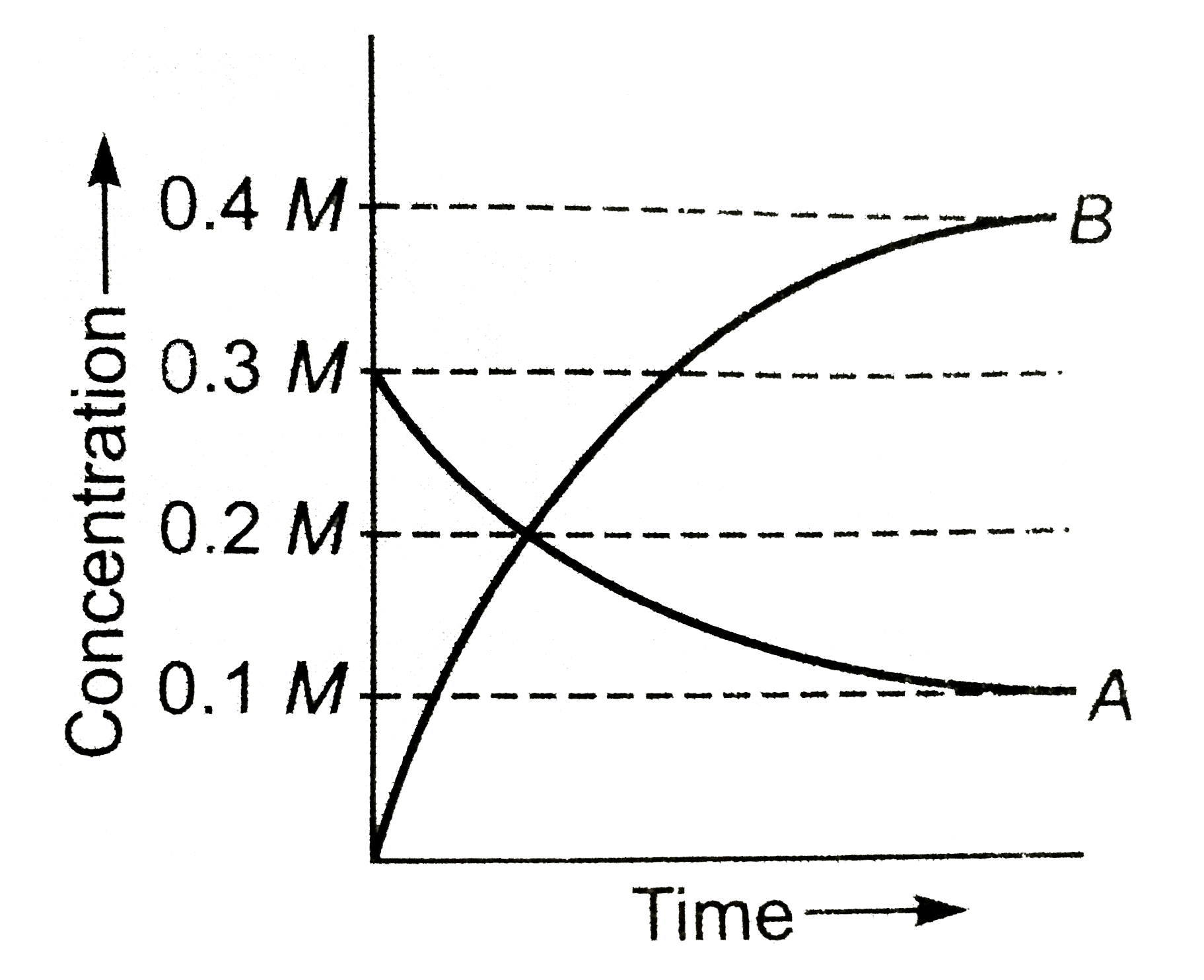
- The figure shows the change in concentration of species A and B as a f...
Text Solution
|
- Using moler concentrations, what is the unit of K(c) for the reaction ...
Text Solution
|
- K(c) = 9 for the reaction, A + B hArrC + D. If A and B are taken in eq...
Text Solution
|
- The equilibrium N(2) (g) + O(2) (g)hArr 2NO (g) is established in a re...
Text Solution
|
- An equilibrium mixture for the reaction 2H(2)S(g) hArr 2H(2)(g) + S(...
Text Solution
|
- What is the unit of K(p) for the reaction ? CS(2)(g)+4H(4)(g)hArrCH(...
Text Solution
|
- N2 and H2 are taken in 1:3 molar ratio in a closed vessel to attained ...
Text Solution
|
- The equilibrium constant, K(p) for the reaction 2SO(2)(g)+O(2)(g)hAr...
Text Solution
|
- For the reaction A(2)(g) + 2B(2)hArr2C(2)(g) the partial pressure of A...
Text Solution
|
- PCl(5)hArrPCl(3)+Cl(2) in the reversible reaction the moles of PCl(5)....
Text Solution
|
- At 1000 K , a sample of pure NO(2) gases decomposes as : 2NO(2)(g)hA...
Text Solution
|
- A 10 litre box contains O3 and O2 at equilibrium at 2000 K. Kp=4xx10^(...
Text Solution
|
- At 527^(@)C, the reaction given below has K(c)=4 NH(3)(g)hArr(1)/(2)...
Text Solution
|
- The value of K(p) fot the reaction 2H(2)O(g) + 2CI(2)(g)hArr4HCI(g) + ...
Text Solution
|
- log Kp/Kc+log RT=0 is a relationship for the reaction :
Text Solution
|
- For the following gases equilibrium, N(2)O(4) (g)hArr2N(2) (g) , K(p)...
Text Solution
|
- consider the following reversible gaseous reaction (at 298 K): (A) N...
Text Solution
|
- 2 moles each of SO(3), CO, SO(2) and CO(2) is taken in a 1 L vessel. I...
Text Solution
|