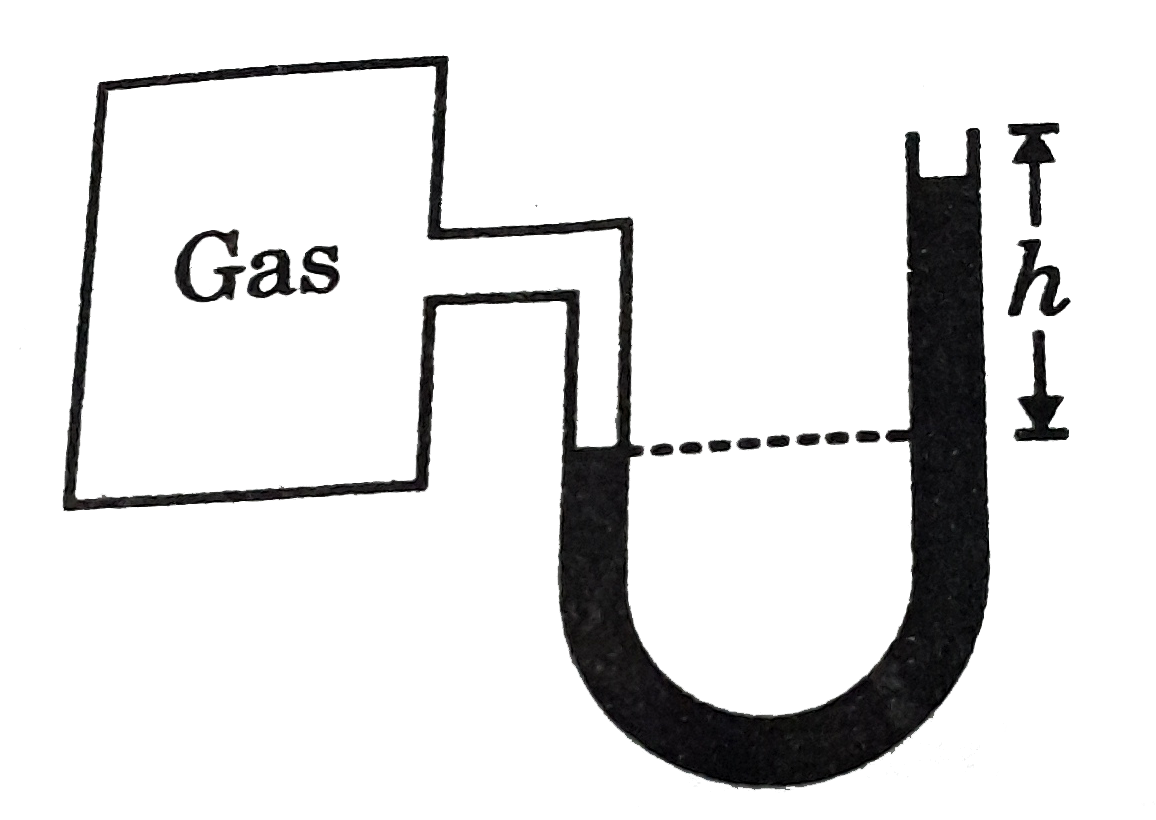Similar Questions
Explore conceptually related problems
Recommended Questions
- If the gas in container gas pressure 77 cm of Hg, then calculate the h...
Text Solution
|
- A manomete is connected to a gas containing bulb. The open arm reads 4...
Text Solution
|
- If the gas in container gas pressure 77 cm of Hg, then calculate the h...
Text Solution
|
- At 27^(@)C a gas under a pressure of 750 mm of Hg occupies a volume of...
Text Solution
|
- Calculate the number of moles of gas present in the container of volum...
Text Solution
|
- When one limb of a manometer is connected to a container filled with a...
Text Solution
|
- A manometer is connected to gas container. Then the mercury level rise...
Text Solution
|
- 71 g mol^(-1)अणुभार की गैस को 30^(@)C पर एक बंद पात्र में रखा गया ...
Text Solution
|
- An open-end manometer was used to determine the pressure of a gas pres...
Text Solution
|