Similar Questions
Explore conceptually related problems
Recommended Questions
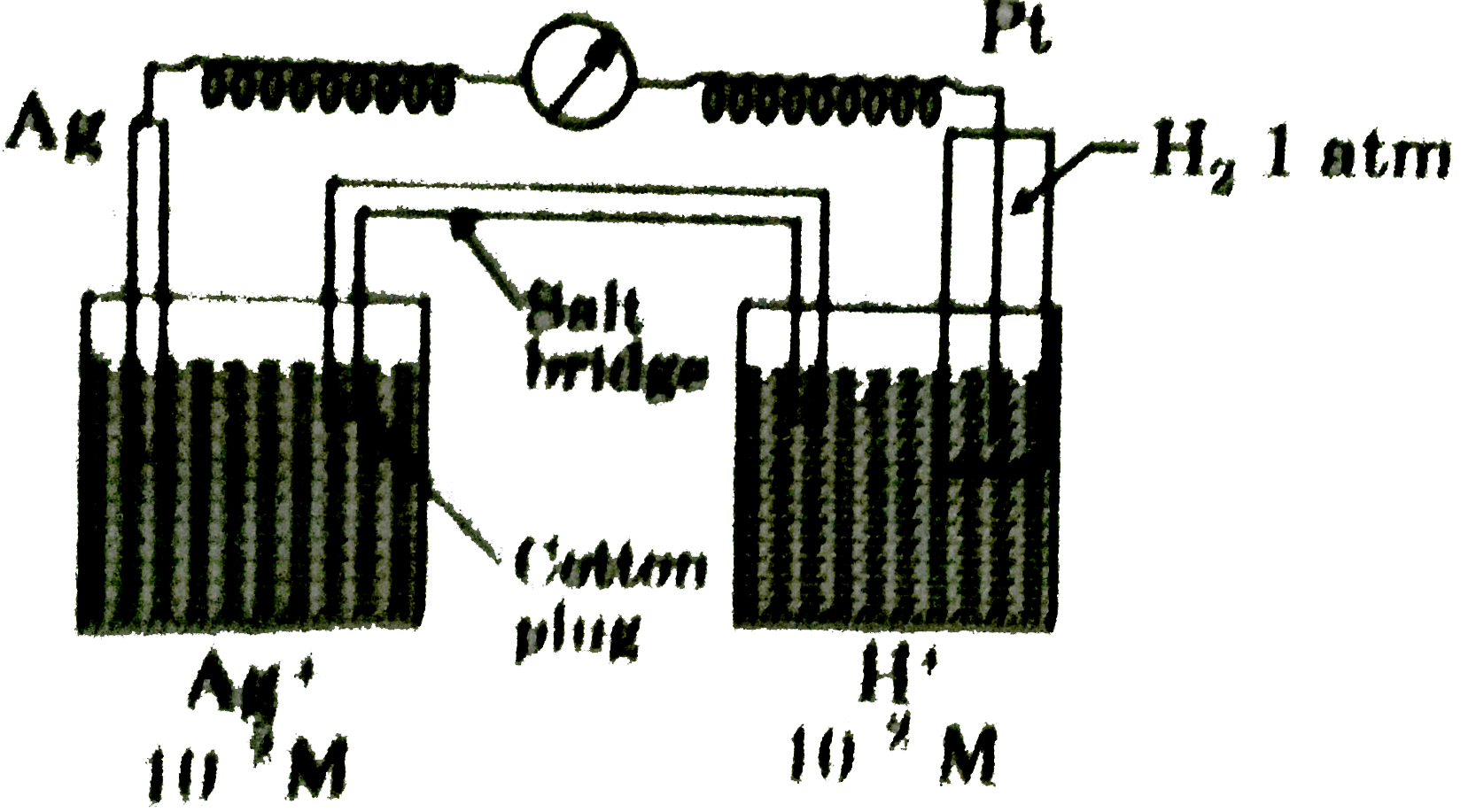
- Calculate emf of given cell at 25^(@)C: [Given: E(Ag^(+)//Ag)^(@)...
Text Solution
|
- Calculate emf of given cell at 25^(@)C : [Given: E(Ag^(+)//Ag)^(@)) = ...
Text Solution
|
- 25^@C पर Ag^(+)(aq) के किस सान्द्रण पर निम्नलिखित सैल का EMF शुन्य हो...
Text Solution
|
- Calculate the electrical work (at standard state) obtained from galvan...
Text Solution
|
- Consider the galvanic cell Cu|Cu^(2+)(0.13M)||Ag^(+)(0.01M)|Ag (i) Cal...
Text Solution
|
- For a standard cell, Cu(s)|Cu^(2+)(0.01M)||Ag^(+)(0.02M)|Ag(s) E(C...
Text Solution
|
- For a standard cell, Cu(s)|Cu^(2+)(0.01M)||Ag^(+)(0.02M)|Ag(s) E(C...
Text Solution
|
- Calculate standard free energy change for the reaction 2Ag+2H^(+)rarrH...
Text Solution
|
- Calculate the emf of given cell Ag| underset(0.001M)(AgNO(3)) || under...
Text Solution
|