A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Exercise 3|65 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Additional Problems for Self practice (APSP)|30 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Exercise 1|65 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Advanced Level Problems (Part-3)(Stage-5)|2 VideosGASEOUS STATE
RESONANCE|Exercise PHYSICAL CHMISTRY (Gaseous State)|47 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS -Exercise 2
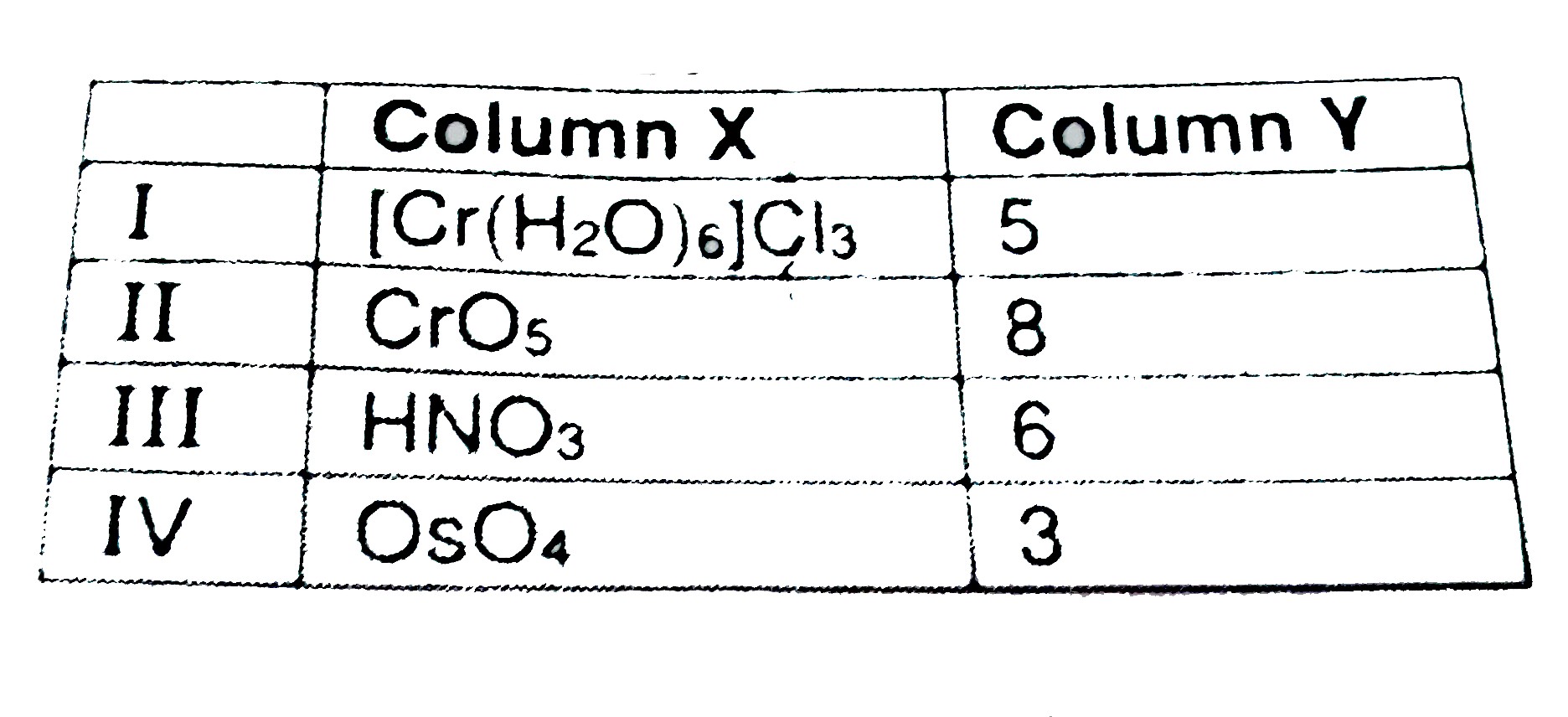
- Match the compounds of column X with oxidation state of central atom i...
Text Solution
|
- Standard reduction electrode potential of Zn^(2+)//Zn is -0.76V. This ...
Text Solution
|
- Of the ions Zn^(2+),Ni^(2+) and Cr^(3+) (atomic number Zn=30,Ni=28,Cr=...
Text Solution
|
- which of the following group of ion is paramagnetic in nature:
Text Solution
|
- Which forms interstitital compouds?
Text Solution
|
- When H(2)O(2) is added to a acidified solution of K(2)Cr(2)O(7):
Text Solution
|
- Sodium thiosulphate is used in photography because of its:
Text Solution
|
- High increase in population is due to:
Text Solution
|
- Which of the following is not an actinide?
Text Solution
|
- The correct statement(s) from among the following is/are? (i). all t...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- Total number of 3d-series transition elements contain either 3d^(1) or...
Text Solution
|
- How many of the following show variable oxidation states in their comp...
Text Solution
|
- Magnetic moment value for a d-block ion is 4.90 BM determine value of ...
Text Solution
|
- Determine total number of unpaired electrons in following ions Ti^(3...
Text Solution
|
- An element of I^(st) transition series X^(+3) have highest magnetic mo...
Text Solution
|
- Chromite ore is processed through the following sequence In this ...
Text Solution
|
- KMnO(4)underset(R.A.)overset(H^(+))toMn^(x) KMnO(4)underset(R.A.)ove...
Text Solution
|
- The no. of electrons satisfying n+l=7 for Lu (atomic number:71)
Text Solution
|
- Correct statements about transiton metals are that they:
Text Solution
|
 .
.