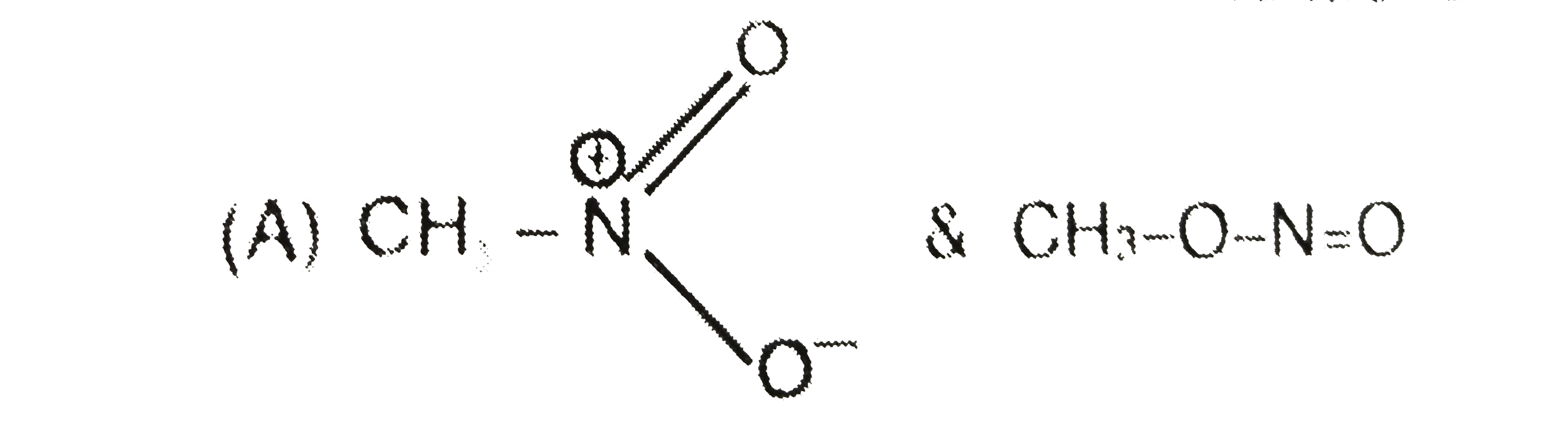
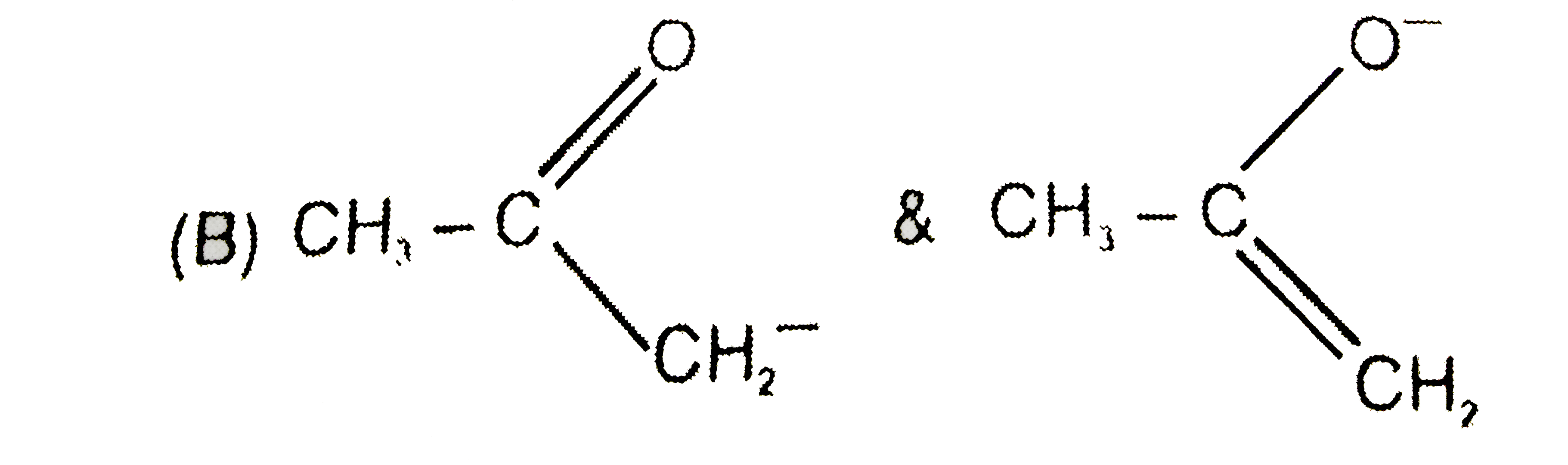
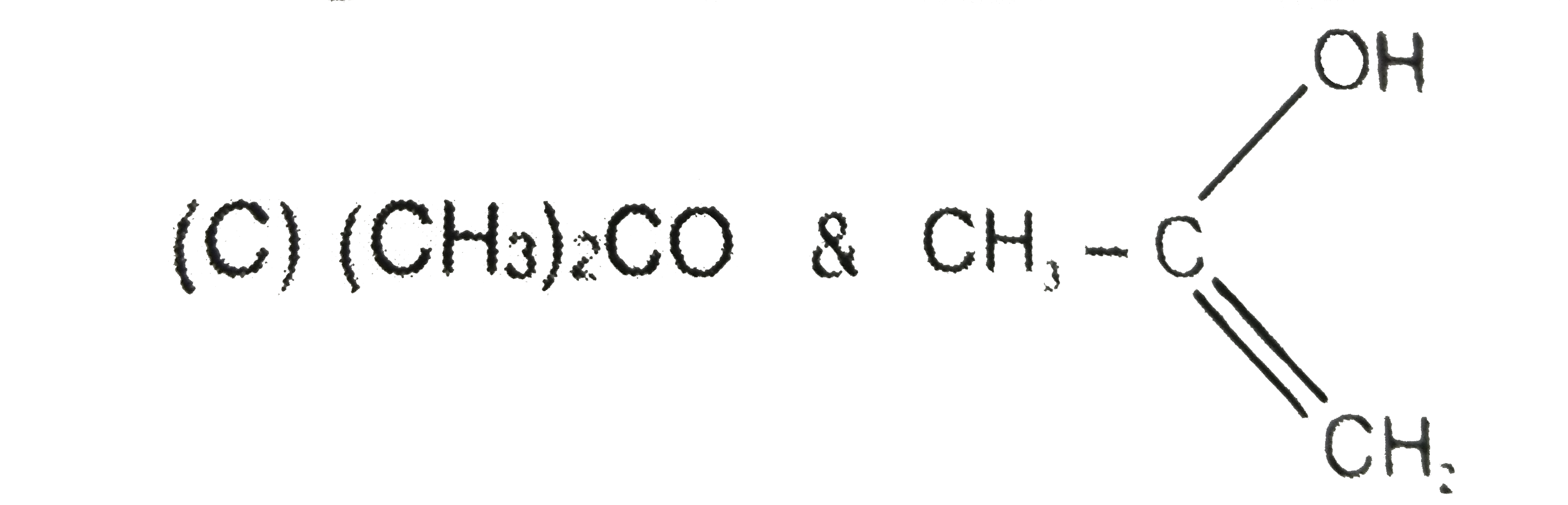A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY-I
RESONANCE|Exercise EXERCISE-1 PART-III (MATCH THE COLUMN)|1 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE|Exercise EXERCISE-2 PART-I|20 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE|Exercise EXERCISE-1|16 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Part-III: Section-5: Matching List Type|1 VideosHYDROGEN AND ITS COMPOUNDS
RESONANCE|Exercise INORGANIC CHEMISTRY(Hydrogen & its compunds Y environment chemistry)|33 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-GENERAL ORGANIC CHEMISTRY-I-EXERCISE-1 PART-II
- Which of the following is not acceptable as resonating structure :
Text Solution
|
- Which of the following pair is not pair of resonating structures ?
Text Solution
|
- Which of the following structures are resonance structures ?
Text Solution
|
- Among the given sets, which represents the resonating structure?
Text Solution
|
- In which of the following resonance is not possible ?
Text Solution
|
- Which one of the following is least stable resonating structure ?
Text Solution
|
- Which of the following resonating structure is the least contributing ...
Text Solution
|
- HNCO (isocyanic acid) has following resonating structures : underset...
Text Solution
|
- The correct stability order of the following resonanating structures i...
Text Solution
|
- Which is the most stable resonating structure ?
Text Solution
|
- Which of the following group show +Meffect ?
Text Solution
|
- Which of the following group show -m effect?
Text Solution
|
- Which of the following group show +M and -l effect?
Text Solution
|
- Which of the following group shown +mgt - I effect?
Text Solution
|
- Which of the following group show -m and -I effect?
Text Solution
|
- +m and +I both effects are shown by:
Text Solution
|
- The weakest +m group of the given species by:
Text Solution
|
- Maximum extent of steric inhibition of resonance can be expected in
Text Solution
|
- In hyperconjugation there is overlap between :
Text Solution
|
- Which of the following cannot exhibit hypercojugation -
Text Solution
|